Titration of Acetic Acid CH3COOH 1) Record the exact Molarity of the freshly prepared solution of NaOH by reading the label on the bottle, include all digits provided. This is the solution that will be in the buret. MB = 0.0500 M 2) Record the exact volume of acid CH3COOH that was pipetted into the Erlenmeyer mL. Note the concentration is unknown and what you will solve for. flask 10.00 (This was diluted with 50 mL of water in the Erlenmeyer flask) Trial 1 Trial 2 Trial 3 Final base (mL) 30.46 30.98 29.88 Initial base (mL)__ 0.45 0.05 0.04 Total base (mL) *Molarity (M) CH COOH *Use stoichiometry to determine the molarity of your CH,COOH solution from each of trial. Show all work including units for one. Calculate Average Molarity
Titration of Acetic Acid CH3COOH 1) Record the exact Molarity of the freshly prepared solution of NaOH by reading the label on the bottle, include all digits provided. This is the solution that will be in the buret. MB = 0.0500 M 2) Record the exact volume of acid CH3COOH that was pipetted into the Erlenmeyer mL. Note the concentration is unknown and what you will solve for. flask 10.00 (This was diluted with 50 mL of water in the Erlenmeyer flask) Trial 1 Trial 2 Trial 3 Final base (mL) 30.46 30.98 29.88 Initial base (mL)__ 0.45 0.05 0.04 Total base (mL) *Molarity (M) CH COOH *Use stoichiometry to determine the molarity of your CH,COOH solution from each of trial. Show all work including units for one. Calculate Average Molarity
Chapter9: Acids, Bases, And Salts
Section: Chapter Questions
Problem 9.101E
Related questions
Question
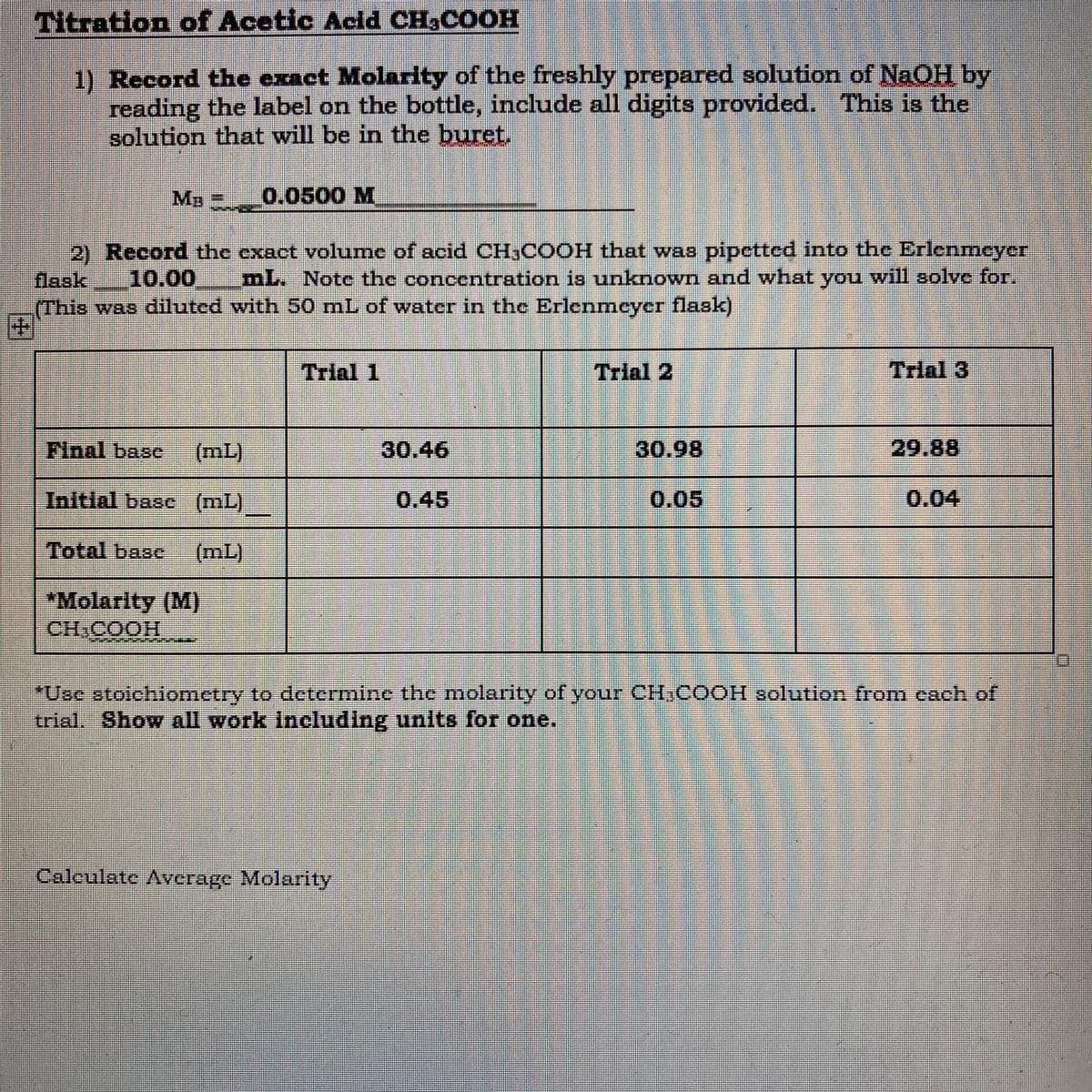
Transcribed Image Text:Titration of Acetic Acid CH3COOH
1) Record the exact Molarity of the freshly prepared solution of NaOH by
reading the label on the bottle, include all digits provided. This is the
solution that will be in the buret.
MB 0.0500 M
2) Record the exact volume of acid CH,COOH that was pipetted into the Erlenmeyer
10.00
aak
mL. Note the concentration is unknown and what you will solve for.
(This was diluted with 50 mL of water in the Erlenmeyer flask)
Trial 1
Trial 2
Trial 3
Final base
(mL)
30.46
30.98
29.88
Initial basc (mL)
0.45
0.05
0.04
Total base
(mL)
*Molarity (M)
HOOD HO
"Use atoichiometry to determine the molarity of your CIHCOOH solution fromn each of
trial. Show all work Including units for one.
Caleulate Average Molarity
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning