• Part A 190 mL of a 31.8 % (m/v) LINO3 solution Express your answer using two significant figures. ? m = Submit Request Answer • Part B 460 mL of a 1.8 % (m/v) KOH solution Express your answer using two significant figures. ? to
• Part A 190 mL of a 31.8 % (m/v) LINO3 solution Express your answer using two significant figures. ? m = Submit Request Answer • Part B 460 mL of a 1.8 % (m/v) KOH solution Express your answer using two significant figures. ? to
Principles of Instrumental Analysis
7th Edition
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
ChapterA1: Evaluation Of Analytical Data
Section: Chapter Questions
Problem A1.22QAP
Related questions
Question
Transcribed Image Text:Course Home
Ô https://openvellum.ecollege.com/course.html?courseld=16516363&OpenVellumHMAC=6607995b61ef1ebc798fc86a6680d4f.
Course Home
Problem 9.42 - Enhanced - with Feedback
9 of 11
Syllabus
II Review I Constants I Periodic Table
Scores
Calculate the amount of solute needed to prepare
the following solutions.
Express your answer using two significant figures.
еТext
?
Document Sharing
User Settings
m =
g
Course Tools
>
Submit
Request Answer
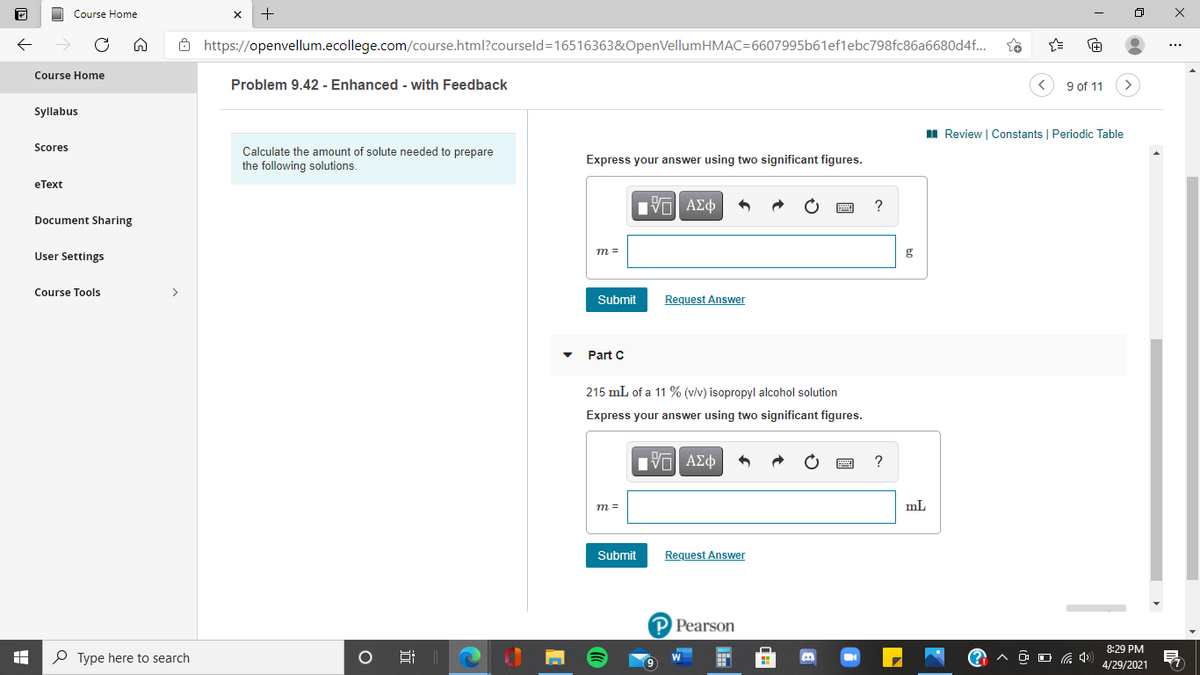
• Part C
215 mL of a 11 % (v/v) isopropyl alcohol solution
Express your answer using two significant figures.
m =
mL
Submit
Request Answer
P Pearson
8:29 PM
P Type here to search
O O G 4)
4/29/2021
近
Transcribed Image Text:Course Home
Ô https://openvellum.ecollege.com/course.html?courseld=16516363&OpenVellumHMAC=6607995b61ef1ebc798fc86a6680d4f.
Course Home
Problem 9.42 - Enhanced - with Feedback
9 of 11
Syllabus
I Review | Constants | Periodic Table
Scores
Calculate the amount of solute needed to prepare
the following solutions.
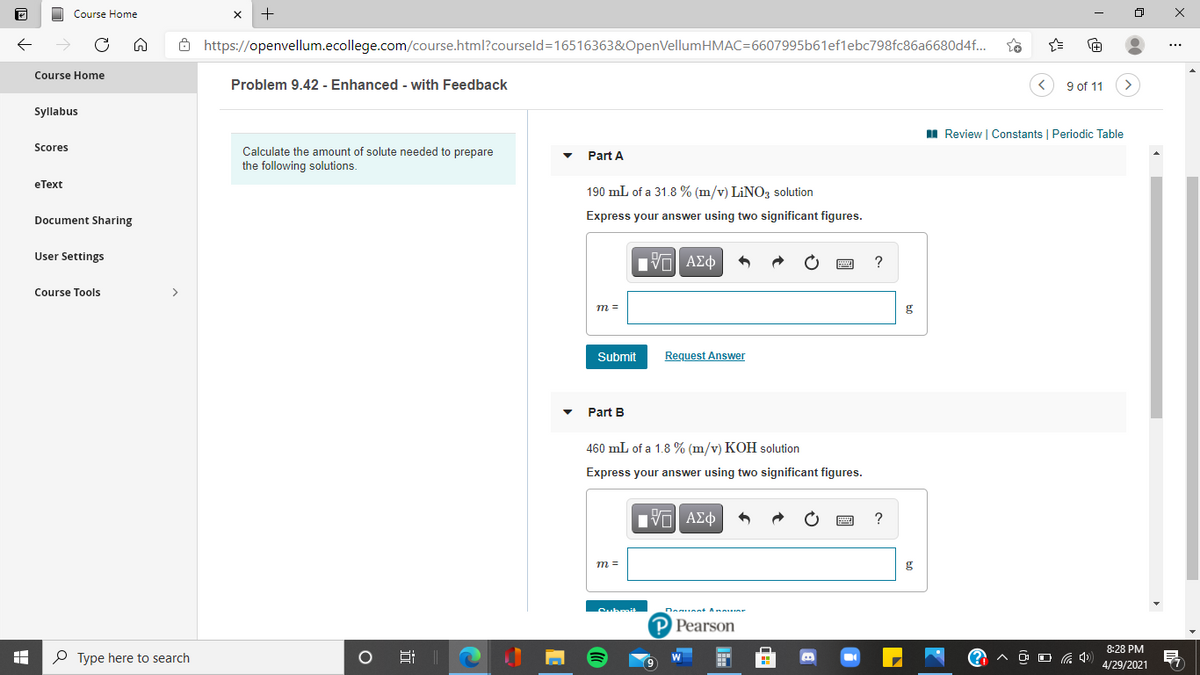
Part A
еТext
190 mL of a 31.8 % (m/v) LINO, solution
Document Sharing
Express your answer using two significant figures.
User Settings
Course Tools
>
m =
g
Submit
Request Answer
Part B
460 mL of a 1.8 % (m/v) KOH solution
Express your answer using two significant figures.
т
g
P Pearson
8:28 PM
P Type here to search
O O G 4)
4/29/2021
近
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning



Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

