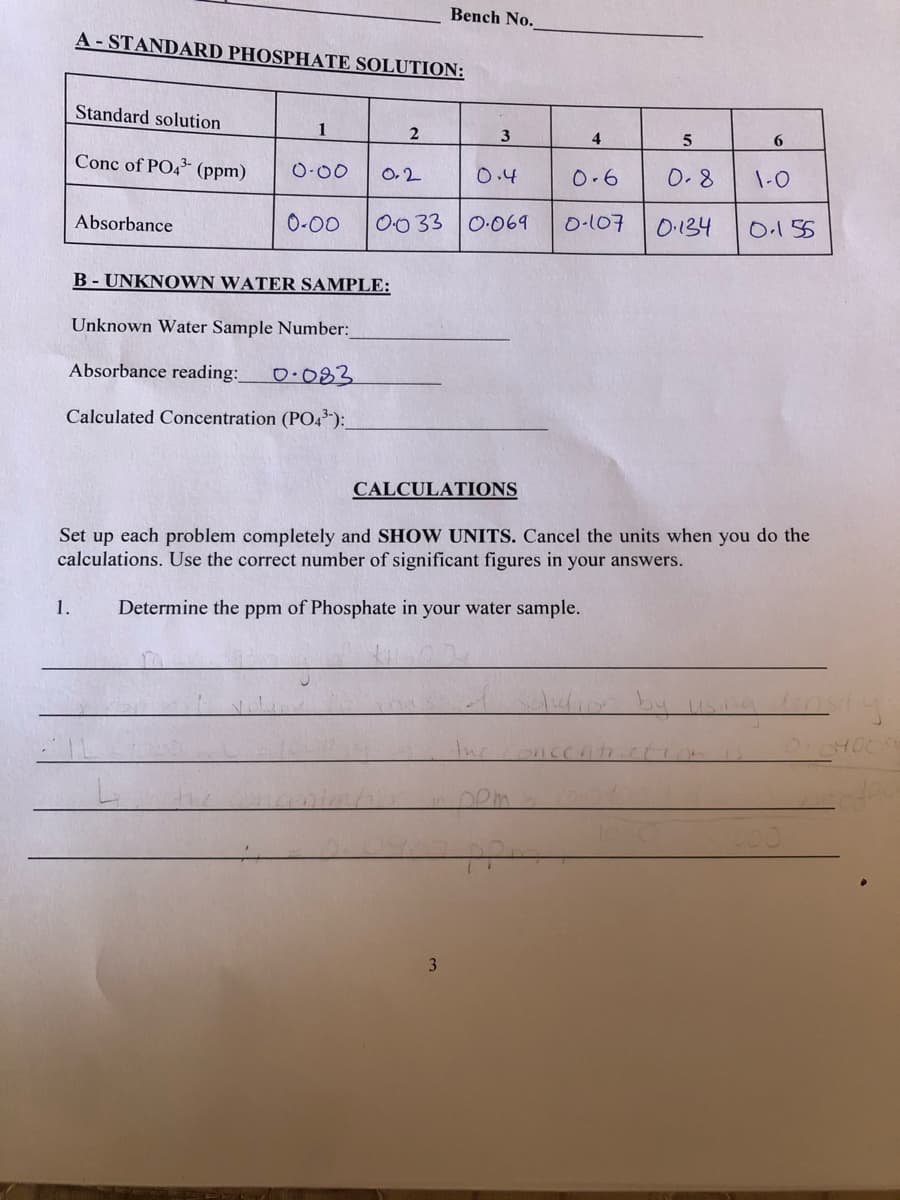
A- STANDARD PHOSPHATE SOLUTION: Standard solution 1 4 6. Conc of PO4 (ppm) O-00 O.2 0.4 0.6 0.8 1-0 Absorbance 0-00 O0 33 0:069 O-107 0.134 0.155 B - UNKNOWN WATER SAMPLE: Unknown Water Sample Number: Absorbance reading: 0.083 Calculated Concentration (P04"): CALCULATIONS Set each problem completely and SHOW UNITS. Cancel the units when you do the up calculations. Use the correct number of significant figures in your answers. 1. Determine the ppm of Phosphate in your water sample. by us Inconcent
A- STANDARD PHOSPHATE SOLUTION: Standard solution 1 4 6. Conc of PO4 (ppm) O-00 O.2 0.4 0.6 0.8 1-0 Absorbance 0-00 O0 33 0:069 O-107 0.134 0.155 B - UNKNOWN WATER SAMPLE: Unknown Water Sample Number: Absorbance reading: 0.083 Calculated Concentration (P04"): CALCULATIONS Set each problem completely and SHOW UNITS. Cancel the units when you do the up calculations. Use the correct number of significant figures in your answers. 1. Determine the ppm of Phosphate in your water sample. by us Inconcent
Chapter27: Molecular Fluorescence Spectroscopy
Section: Chapter Questions
Problem 27.11QAP
Related questions
Question
Transcribed Image Text:Bench No.
A - STANDARD PHOSPHATE SOLUTION:
Standard solution
1
3
4
6.
Conc of PO43- (ppm)
O-00
O,2
0.4
0-6
0.8
1-0
Absorbance
0-00
00 33 0:069
O-107
O.134
O.155
B - UNKNOWN WATER SAMPLE:
Unknown Water Sample Number:
Absorbance reading: O 083
Calculated Concentration (PO43-):
CALCULATIONS
Set up each problem completely and SHOW UNITS. Cancel the units when you do the
calculations. Use the correct number of significant figures in your answers.
1.
Determine the ppm of Phosphate in your water sample.
by usa
3

Transcribed Image Text:2.
Determine the number of moles of Phosphate in your water sample.
136.036
090
Mi
3.
Determine the ppm of Phosphorus in your original water sample.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning