Data Drawer Number Part I. Standardization of the Sodium Hydroxide Solution Mass of weighing bottle + sample 12-5252g Mass of weighing bottle sample 99932g Mass of H2C20. 2 H20 2.5320g Volume of H2C2O4 solution 250.00 mL Run Number 1 3 Volume of oxalic acid used 25.00 mL 25.00 mL 25.00 mL 25.00 mL NaOH buret: final reading 42.10mL 42.20mL 212-25L 42.502 NAOH buret: initial reading 0.20ML D.15ML 0.40mL 0.55ML Volume of NaOH used 211.902 212.05wL 41.85mL
Data Drawer Number Part I. Standardization of the Sodium Hydroxide Solution Mass of weighing bottle + sample 12-5252g Mass of weighing bottle sample 99932g Mass of H2C20. 2 H20 2.5320g Volume of H2C2O4 solution 250.00 mL Run Number 1 3 Volume of oxalic acid used 25.00 mL 25.00 mL 25.00 mL 25.00 mL NaOH buret: final reading 42.10mL 42.20mL 212-25L 42.502 NAOH buret: initial reading 0.20ML D.15ML 0.40mL 0.55ML Volume of NaOH used 211.902 212.05wL 41.85mL
Chapter10: Potentiometry And Redox Titrations
Section: Chapter Questions
Problem 7P
Related questions
Question
Using the data, calculate the molarity of sodium hydroxide solution for each run.
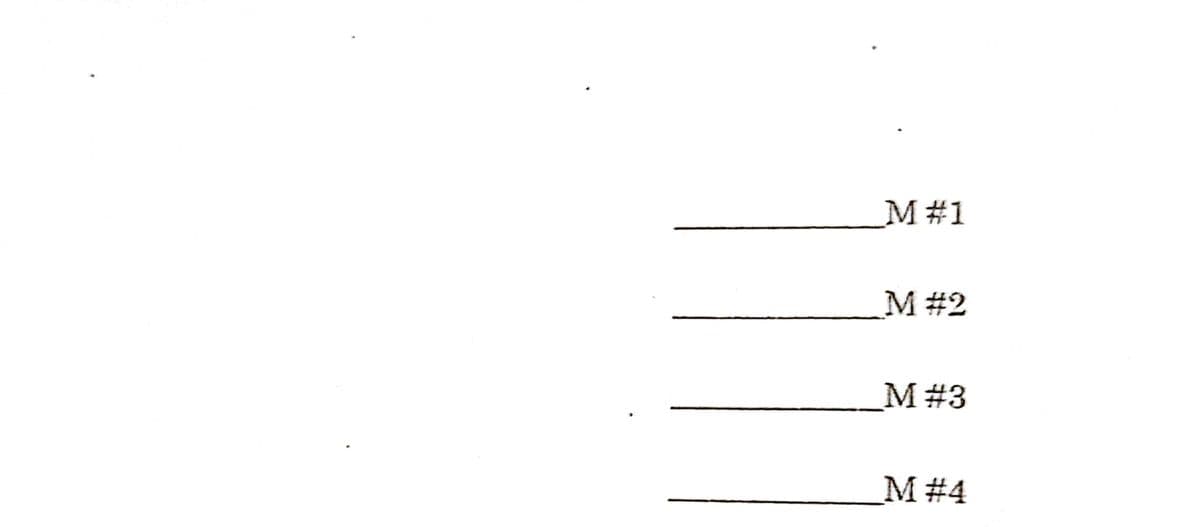
Transcribed Image Text:M#1
M #2
M #3
M #4
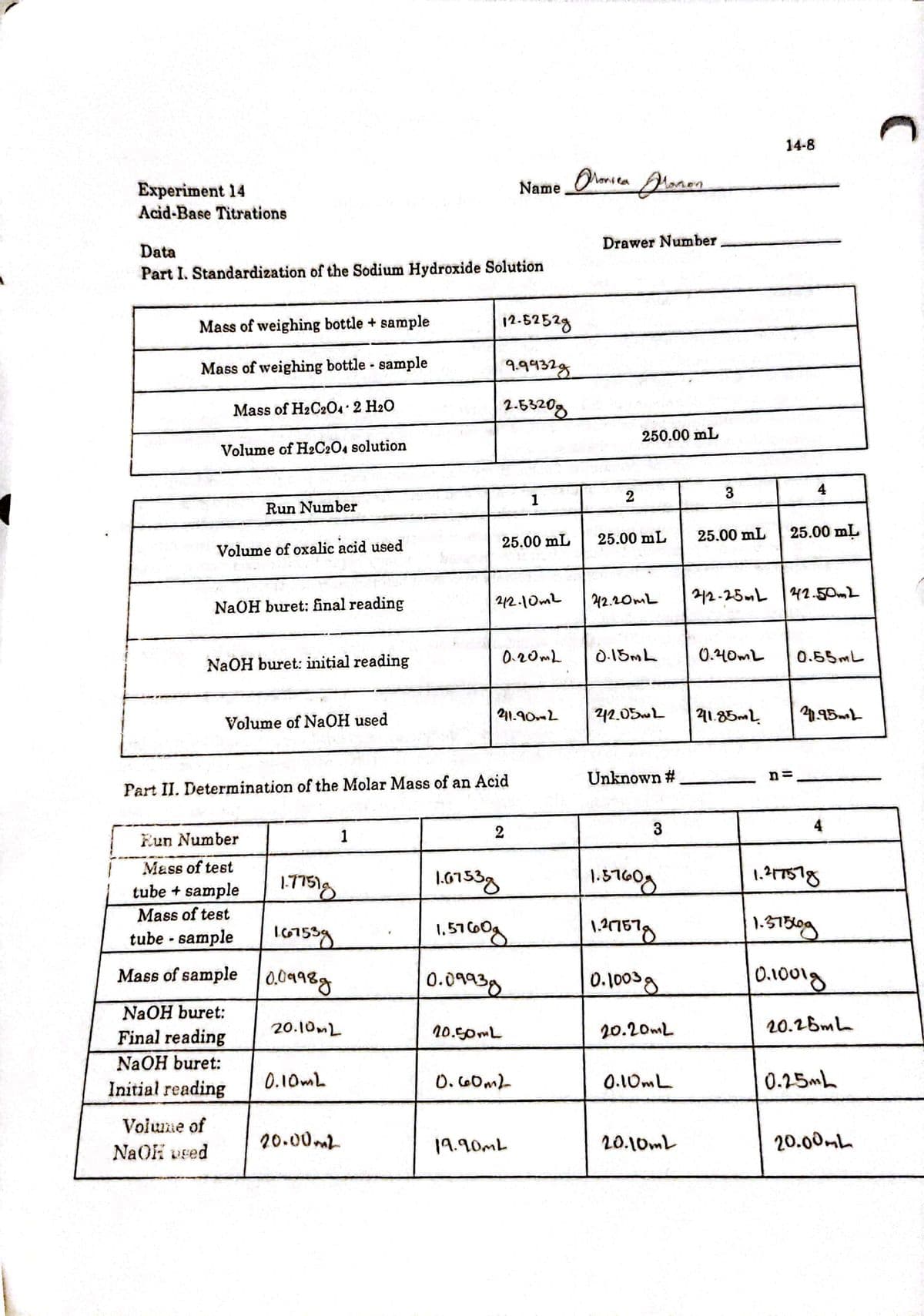
Transcribed Image Text:14-8
Experiment 14
Name Olonsea
Acid-Base Titrations
Drawer Number
Data
Part I. Standardization of the Sodium Hydroxide Solution
Mass of weighing bottle + sample
12.5252g
Mass of weighing bottle - sample
9432g
Mass of H2C20 2 H20
2.5320g
250.00 mL
Volume of H2C204 solution
2
3
4
1
Run Number
25.00 mL
25.00 mL
25.00 mL
25.00 mL
Volume of oxalic acid used
42.20mL
212-25mL 42-50m2
NaOH buret: final reading
212.10mL
0.20mL
D.15ML
0.40mL
0.55ML
NaOH buret: initial reading
211.90L
242.05wL
41.85mL
Volume of NaOH used
Unknown #
n=
Part II. Determination of the Molar Mass of an Acid
2
3
4
Eun Number
1
Mass of test
1.417578
tube + sample
Mass of test
tube - sample
1,5160g
1.S13og
Mass of sample
0,0998g
0.09939
0.1003g
0.1001g
NaOH buret:
20.10ML
20.50ML
20.20mL
20.26ML
Final reading
NaOH buret:
Initial reading
0.10mL
O. 60ml-
0.10ML
0.25mh
Volume of
20.00mL
19.90ML
20.10mL
20.00mL
NaOH vsed
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 7 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you



Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning



Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning
