A group of students titrate a calcium carbonate standard solution repeatedly in five experimental trials. Based on the five trials, the group detemines that the average concentration of the standard is 80.5 ppm with a standard deviation of 0.5 ppm. Using the equation for the Student's t confidence interval found in Appendix 1 of your lab manual and the t-value for a 95% confidence interval, calculate the confidence interval for which there is a 95% chance of finding the "true value" of the standard solution's concentration based on the data. Put your answer in the fomat # lower limit < "true value" < # upper limit. (Appendix 2 has an example that may be helpful.)
A group of students titrate a calcium carbonate standard solution repeatedly in five experimental trials. Based on the five trials, the group detemines that the average concentration of the standard is 80.5 ppm with a standard deviation of 0.5 ppm. Using the equation for the Student's t confidence interval found in Appendix 1 of your lab manual and the t-value for a 95% confidence interval, calculate the confidence interval for which there is a 95% chance of finding the "true value" of the standard solution's concentration based on the data. Put your answer in the fomat # lower limit < "true value" < # upper limit. (Appendix 2 has an example that may be helpful.)
Chapter4: Least-squares And Calibration Methods
Section: Chapter Questions
Problem 4P
Related questions
Question
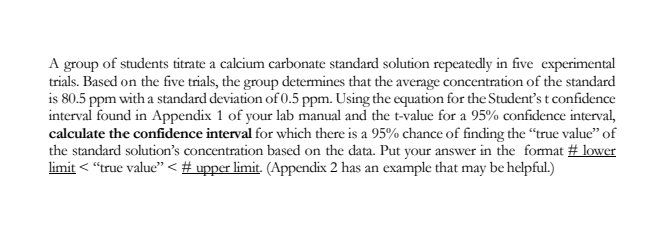
Transcribed Image Text:A group of students titrate a calcium carbonate standard solution repeatedly in five experimental
trials. Based on the five trials, the group determines that the average concentration of the standard
is 80.5 ppm with a standard deviation of 0.5 ppm. Using the equation for the Student's t confidence
interval found in Appendix 1 of your lab manual and the t-value for a 95% confidence interval,
calculate the confidence interval for which there is a 95% chance of finding the "true value" of
the standard solution's concentration based on the data. Put your answer in the format # lower
limit < "true value" < # upper limit. (Appendix 2 has an example that may be helpful.)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you



Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning



Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning