Part A A semipermeable membrane is placed between the following solutions. Which solution will decrease in volume? • View Available Hint(s) O Solution A: 1.81 % (mv) starch O Solution B: 6.89 % (m/v) starch Submit Part B A semipermeable membrane is placed between the following solutions. Which solution will increase in volume? > View Available Hint(s) O Solution C: 7.40 % NaCl Solution D: 14.6 % NaCl Submit Part C A red blood cell has been placed into each of three different solutions. One solution is isotonic to the cell, one solution hypotonic to the cell, and one solution is hypertonic to the cell. Sort each beaker into the appropriate bin based on the reaction in each solution.
Part A A semipermeable membrane is placed between the following solutions. Which solution will decrease in volume? • View Available Hint(s) O Solution A: 1.81 % (mv) starch O Solution B: 6.89 % (m/v) starch Submit Part B A semipermeable membrane is placed between the following solutions. Which solution will increase in volume? > View Available Hint(s) O Solution C: 7.40 % NaCl Solution D: 14.6 % NaCl Submit Part C A red blood cell has been placed into each of three different solutions. One solution is isotonic to the cell, one solution hypotonic to the cell, and one solution is hypertonic to the cell. Sort each beaker into the appropriate bin based on the reaction in each solution.
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter10: Solutions
Section: Chapter Questions
Problem 66QAP: The Henry's law constant for the solubility of radon in water at is 9.57106 M/mm Hg. Radon is...
Related questions
Question
100%
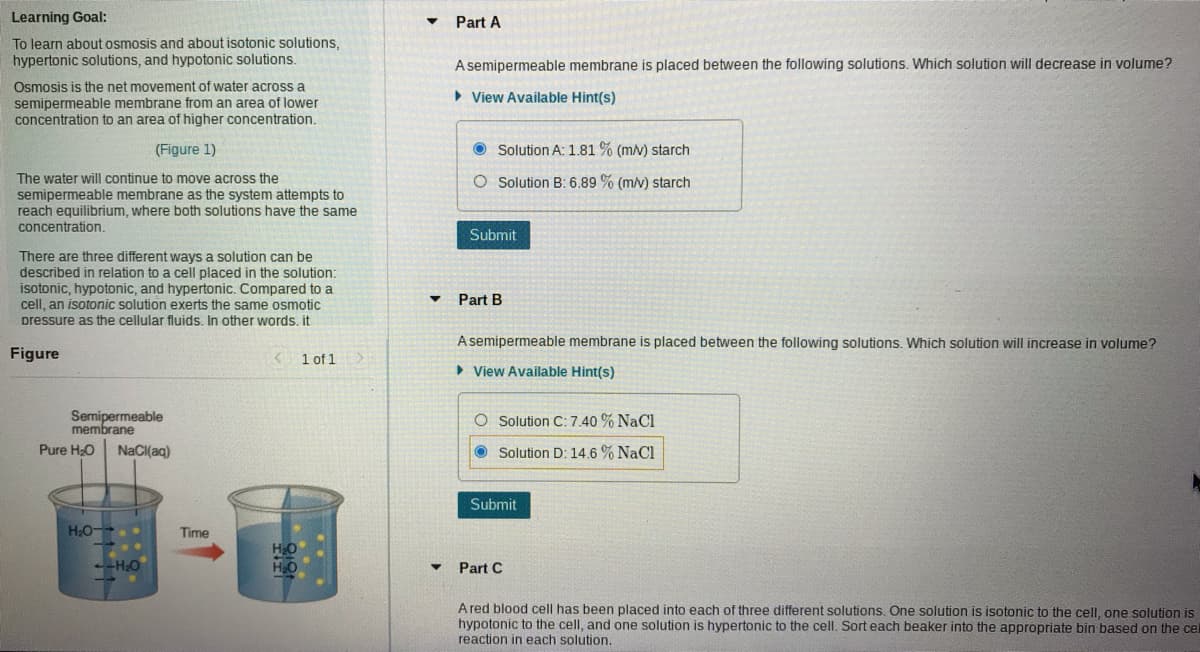
Transcribed Image Text:Learning Goal:
Part A
To learn about osmosis and about isotonic solutions,
hypertonic solutions, and hypotonic solutions.
A semipermeable membrane is placed between the following solutions. Which solution will decrease in volume?
Osmosis is the net movement of water across a
semipermeable membrane from an area of lower
concentration to an area of higher concentration.
• View Available Hint(s)
(Figure 1)
O Solution A: 1.81 % (m/v) starch
The water will continue to move across the
O Solution B: 6.89 % (m/v) starch
semipermeable membrane as the system attempts to
reach equilibrium, where both solutions have the same
concentration.
Submit
There are three different ways a solution can be
described in relation to a cell placed in the solution:
isotonic, hypotonic, and hypertonic. Compared to a
cell, an isotonic solution exerts the same osmotic
pressure as the cellular fluids. In other words. it
Part B
A semipermeable membrane is placed between the following solutions. Which solution will increase in volume?
Figure
< 1 of 1
> View Available Hint(s)
Semipermeable
membrane
O Solution C: 7.40 % NaCl
Pure HO
NaCl(aq)
O Solution D: 14.6 % NaCl
Submit
H2O-
Time
H2O
H2O
-HO
Part C
A red blood cell has been placed into each of three different solutions. One solution is isotonic to the celI, one solution is
hypotonic to the cell, and one solution is hypertonic to the cell. Sort each beaker into the appropriate bin based on the cel
reaction in each solution.
Expert Solution
Step 1
Principle
When a semipermeable membrane is placed between two solutions of different concentrations the solvent flows from the solution with a lower concentration to the solution with a higher concentration. As a result the volume of the solution with lower concentration decreases and the volume of the solution with higher concentration increases.
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning