25Which of the following statements is wrong about water: a. Water has a unique ability to dissolve many organic and inorganic compounds. O b. Water expands when it freezes. This is related to the crystal structure of ice. O. Virtually no water is pure; it can only be made in a laboratory. O d. 80 elements have been detected in seawater. O e. Chlorine, sodium, magnesium and boron are commercially extracted from sea water. O f. None of the above Previous page
25Which of the following statements is wrong about water: a. Water has a unique ability to dissolve many organic and inorganic compounds. O b. Water expands when it freezes. This is related to the crystal structure of ice. O. Virtually no water is pure; it can only be made in a laboratory. O d. 80 elements have been detected in seawater. O e. Chlorine, sodium, magnesium and boron are commercially extracted from sea water. O f. None of the above Previous page
Chemistry & Chemical Reactivity
9th Edition
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter13: Solutions And Their Behavior
Section: Chapter Questions
Problem 22PS: Hydrogen gas has a Henrys law constant of 7.8 104 mol/kgbar at 25 C when dissolving in water. If...
Related questions
Question
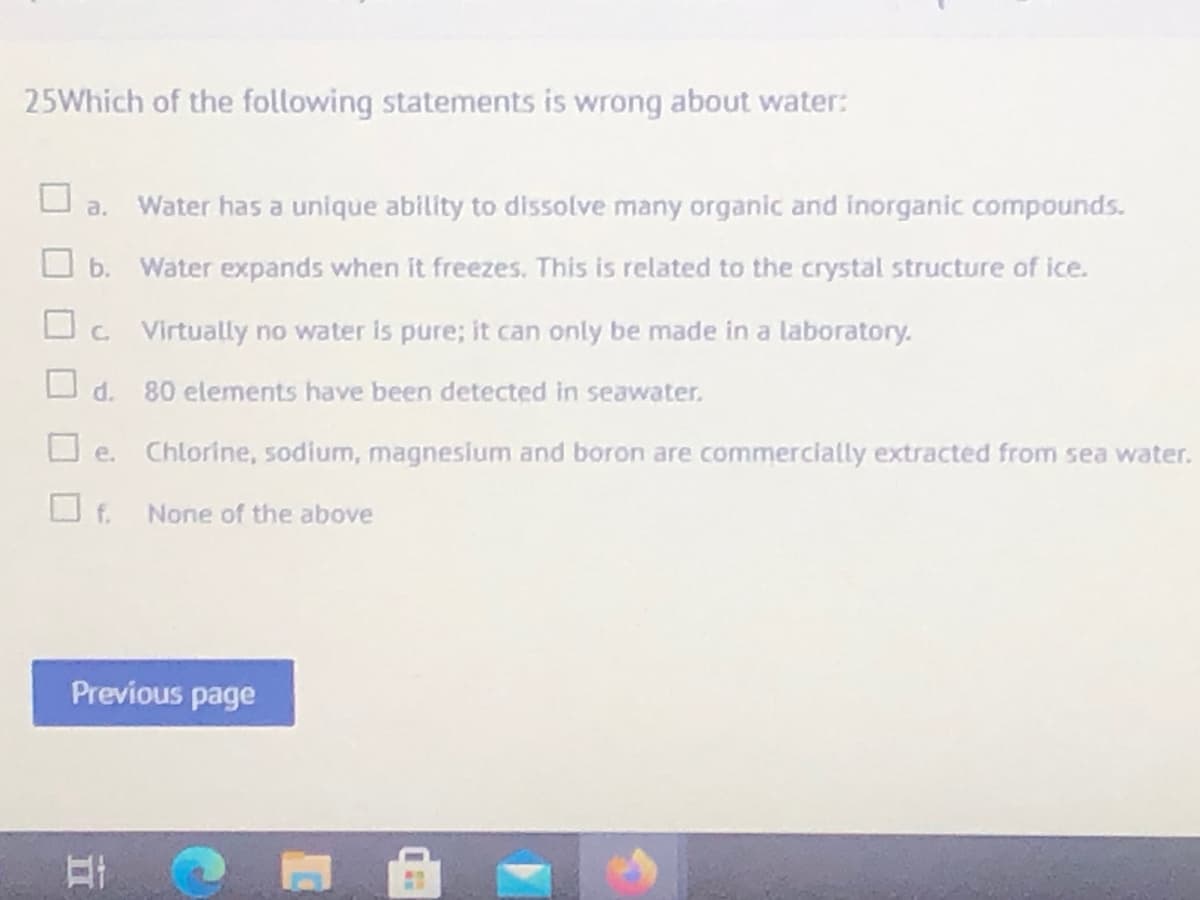
Transcribed Image Text:25Which of the following statements is wrong about water:
a.
Water has a unique ability to dissolve many organic and inorganic compounds.
O b.
Water expands when it freezes. This is related to the crystal structure of ice.
O. Virtually no water is pure; it can only be made in a laboratory.
d.
80 elements have been detected in seawater.
Chlorine, sodium, magneslum and boron are commercially extracted from sea water.
O e.
f.
None of the above
Previous page
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning