Part A A total of 2.00 mol of a compound is allowed to react with water in a foam coffee cup and the reaction produces 107 g of solution. The reaction caused the temperature of the solution to rise from 21.00 to 24.70 °C. What is the enthalpy of this reaction? Assume that no heat is lost to the surroundings or to the coffee cup itself and that the specific heat of the solution is the same as that of pure water. Enter your answer in kilojoules per mole of compound to three significant figures. > View Available Hint(s) VO AE ? ΔΗ. Amol
Part A A total of 2.00 mol of a compound is allowed to react with water in a foam coffee cup and the reaction produces 107 g of solution. The reaction caused the temperature of the solution to rise from 21.00 to 24.70 °C. What is the enthalpy of this reaction? Assume that no heat is lost to the surroundings or to the coffee cup itself and that the specific heat of the solution is the same as that of pure water. Enter your answer in kilojoules per mole of compound to three significant figures. > View Available Hint(s) VO AE ? ΔΗ. Amol
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 1RQ: Define and explain the differences between the following terms. a. law and theory b. theory and...
Related questions
Question
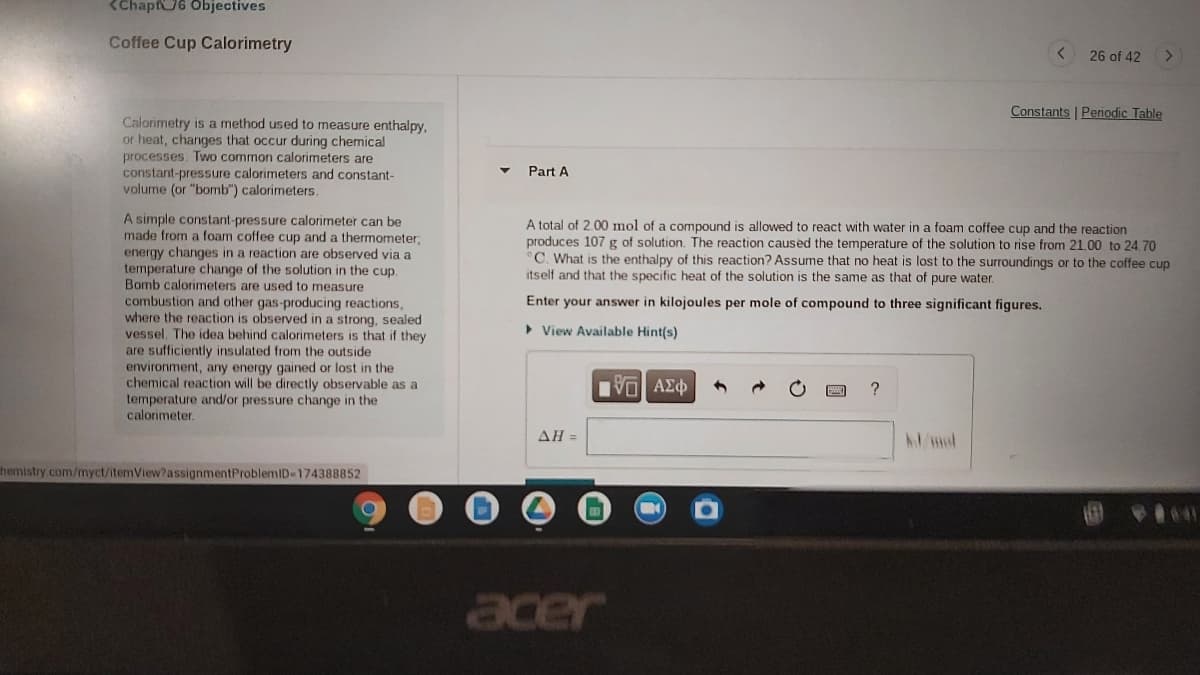
Transcribed Image Text:<Chapf6 Objectives
Coffee Cup Calorimetry
26 of 42
Constants | Periodic Table
Calorimetry is a method used to measure enthalpy.
or heat, changes that occur during chemical
processes. Two common calorimeters are
constant-pressure calorimeters and constant-
volume (or "bomb") calorimeters.
Part A
A simple constant-pressure calorimeter can be
made from a foam coffee cup and a thermometer;
energy changes in a reaction are observed via a
temperature change of the solution in the cup.
Bomb calorimeters are used to
combustion and other gas-producing reactions,
where the reaction is observed in
vessel. The idea behind calorimeters is that if they
are sufficiently insulated from the outside
environment, any energy gained or lost in the
chemical reaction will be directly observable as a
temperature and/or pressure change in the
calorimeter.
A total of 2.00 mol of a compound is allowed to react with water in a foam coffee cup and the reaction
produces 107 g of solution. The reaction caused the temperature of the solution to rise from 21.00 to 24,70
C. What is the enthalpy of this reaction? Assume that no heat is lost to the surroundings or to the coffee cup
itself and that the specific heat of the solution is the same as that of pure water.
measure
Enter your answer in kilojoules per mole of compound to three significant figures.
a strong, sealed
> View Available Hint(s)
?
ΔΗ
Admol
hemistry.com/myct/itemView?assignmentProblemID-174388852
acer
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9780078021558
Author:
Janice Gorzynski Smith Dr.
Publisher:
McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Elementary Principles of Chemical Processes, Bind…
Chemistry
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY