The Octet Rule When main group elements react, they tend to acquire eight outer-shell electrons (ns np®). This is called an octet, and it is a particularly stable configuration. Part A Group Valence To attain octet If the following elements were involved in redox reactions, which noble-gas configuration would they most likely attain? 1 ns' lose one electron Drag the appropriate elements to their respective bins. ns lose two electrons 13 ns np lose three electrons 15 ns np gain three electrons 16 nsnp 17 ns np 18 ns np® rarely gain or lose electrons gain two electrons gain one electron Li AI0P Sc Br Rb Nonmetals in the third period and beyond can break the octet rule because they have vacant d orbitals that allow them to accommodate more than eight outer-shell electrons. Не Ne Ar Kr 2.
The Octet Rule When main group elements react, they tend to acquire eight outer-shell electrons (ns np®). This is called an octet, and it is a particularly stable configuration. Part A Group Valence To attain octet If the following elements were involved in redox reactions, which noble-gas configuration would they most likely attain? 1 ns' lose one electron Drag the appropriate elements to their respective bins. ns lose two electrons 13 ns np lose three electrons 15 ns np gain three electrons 16 nsnp 17 ns np 18 ns np® rarely gain or lose electrons gain two electrons gain one electron Li AI0P Sc Br Rb Nonmetals in the third period and beyond can break the octet rule because they have vacant d orbitals that allow them to accommodate more than eight outer-shell electrons. Не Ne Ar Kr 2.
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter12: Chemical Bonding
Section: Chapter Questions
Problem 47CR: Which of the following statements is correct and provides the best explanation when removing the...
Related questions
Question
tell the right answer for both 7ab ASAP
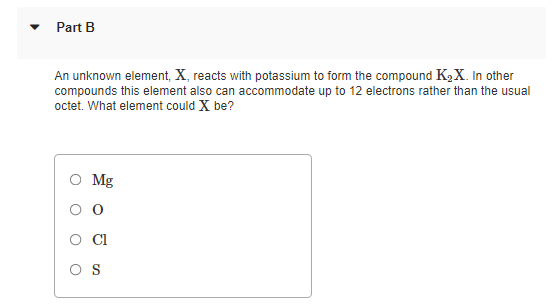
Transcribed Image Text:Part B
An unknown element, X, reacts with potassium to form the compound K2X. In other
compounds this element also can accommodate up to 12 electrons rather than the usual
octet. What element could X be?
O Mg
Cl
O S
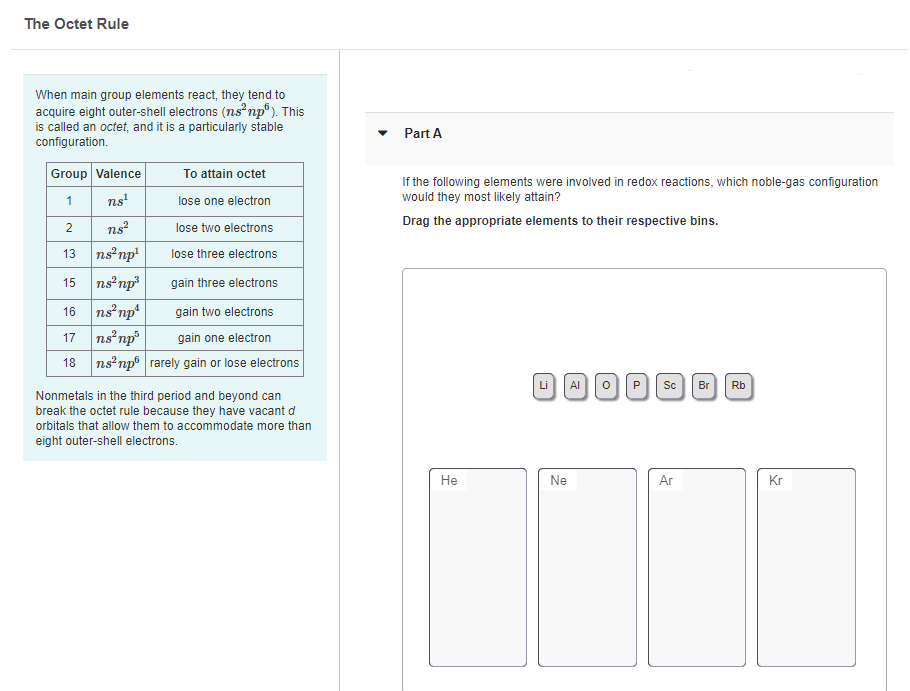
Transcribed Image Text:The Octet Rule
When main group elements react, they tend to
acquire eight outer-shell electrons (ns np°). This
is called an octet, and it is a particularly stable
configuration.
Part A
Group Valence
To attain octet
If the following elements were involved in redox reactions, which noble-gas configuration
would they most likely attain?
1
ns'
lose one electron
Drag the appropriate elements to their respective bins.
ns?
lose two electrons
ns np'
ns np
16 ns np
ns np
18 ns np® rarely gain or lose electrons
13
lose three electrons
15
gain three electrons
gain two electrons
17
gain one electron
Li Al 0P
Sc Br
Rb
Nonmetals in the third period and beyond can
break the octet rule because they have vacant d
orbitals that allow them to accommodate more than
eight outer-shell electrons.
Не
Ne
Ar
Kr
2.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning


Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning


Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning