Part A An experiment measures the temperature of a 300 g substance while steadily supplying heat to it. (Figure 1) shows the results of the experiment. What is the specific heat of the solid phase? Express your answer to two significant figures and include the appropriate units. • View Available Hint(s) HA C, = Value Units Figure 1 of 1 Submit Q (kJ) Part B 200 What is the specific heat of the liquid phase? 160 Express your answer to two significant figures and include the appropriate units. 120 > View Available Hint(s) 80 40 TC) 80 Value Units -40 0. 40
Part A An experiment measures the temperature of a 300 g substance while steadily supplying heat to it. (Figure 1) shows the results of the experiment. What is the specific heat of the solid phase? Express your answer to two significant figures and include the appropriate units. • View Available Hint(s) HA C, = Value Units Figure 1 of 1 Submit Q (kJ) Part B 200 What is the specific heat of the liquid phase? 160 Express your answer to two significant figures and include the appropriate units. 120 > View Available Hint(s) 80 40 TC) 80 Value Units -40 0. 40
Chapter1: Temperature And Heat
Section: Chapter Questions
Problem 48P: A person taking a reading of the temperature in a freezer in Celsius makes two mistakes: first...
Related questions
Question
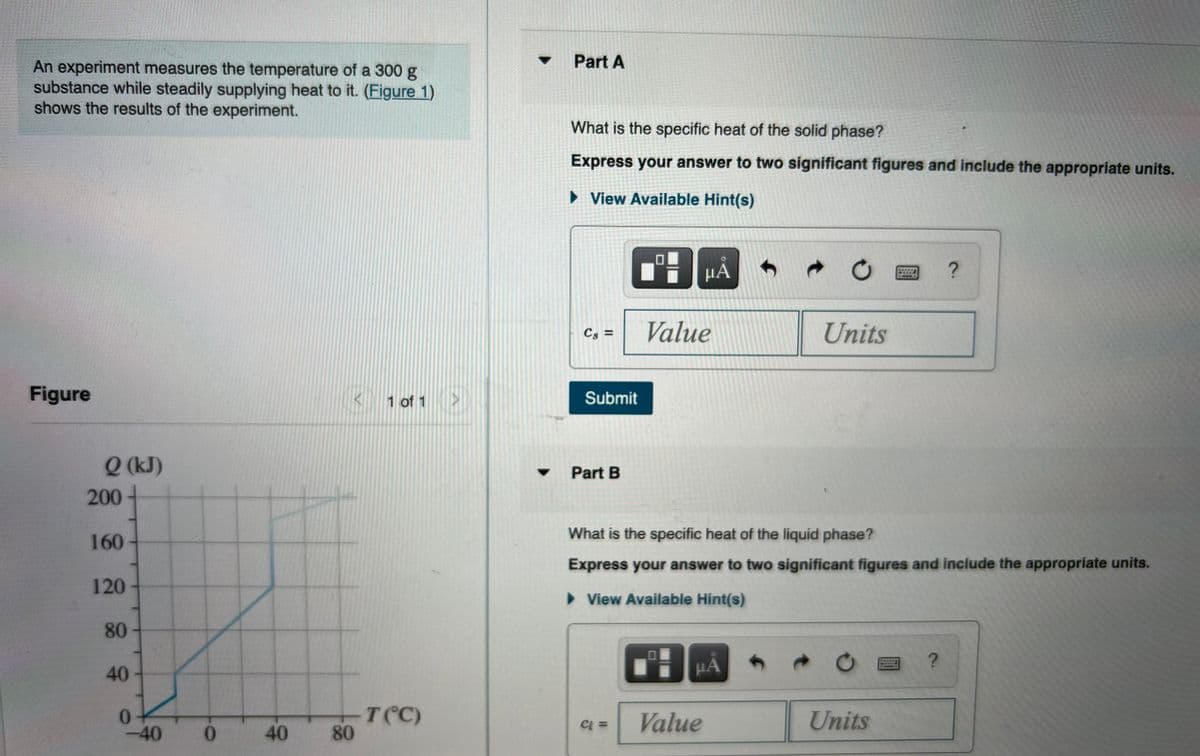
Transcribed Image Text:Part A
An experiment measures the temperature of a 300 g
substance while steadily supplying heat to it. (Figure 1)
shows the results of the experiment.
What is the specific heat of the solid phase?
Express your answer to two significant figures and include the appropriate units.
> View Available Hint(s)
Cs =
Value
Units
Figure
K 1 of 1 >
Submit
Q (kJ)
Part B
200
What is the specific heat of the liquid phase?
160
Express your answer to two significant figures and include the appropriate units.
120
> View Available Hint(s)
80
HA
ww.g
40
μΑ
T(C)
80
Value
Units
-40
2.
40
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Recommended textbooks for you


An Introduction to Physical Science
Physics
ISBN:
9781305079137
Author:
James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar Torres
Publisher:
Cengage Learning


An Introduction to Physical Science
Physics
ISBN:
9781305079137
Author:
James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar Torres
Publisher:
Cengage Learning