Part A Cathode Anode Potential (V) Cu Zn 1.102 Cu Fe 0.366 Cu Al 1.992 Part B Potential (V) Solution 2 1.052 Solution 3 1.001 Solution 4 0.951 Unknown 1.028 Part C Potential (V) Solution 2 0.051 Solution 3 0.106 Solution 4 0.148
Part A Cathode Anode Potential (V) Cu Zn 1.102 Cu Fe 0.366 Cu Al 1.992 Part B Potential (V) Solution 2 1.052 Solution 3 1.001 Solution 4 0.951 Unknown 1.028 Part C Potential (V) Solution 2 0.051 Solution 3 0.106 Solution 4 0.148
Physical Chemistry
2nd Edition
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Ball, David W. (david Warren), BAER, Tomas
Chapter7: Equilibria In Multiple-component Systems
Section: Chapter Questions
Problem 7.57E
Related questions
Question
Explain why the cell potential of the galvanic cells in this experiment decreases over time.
Part A - Determination of Relative Reduction Potentials
Part B - Effect of Differing Concentrations on Cell Potential
Part C - Cell Potential of a Concentration Cell
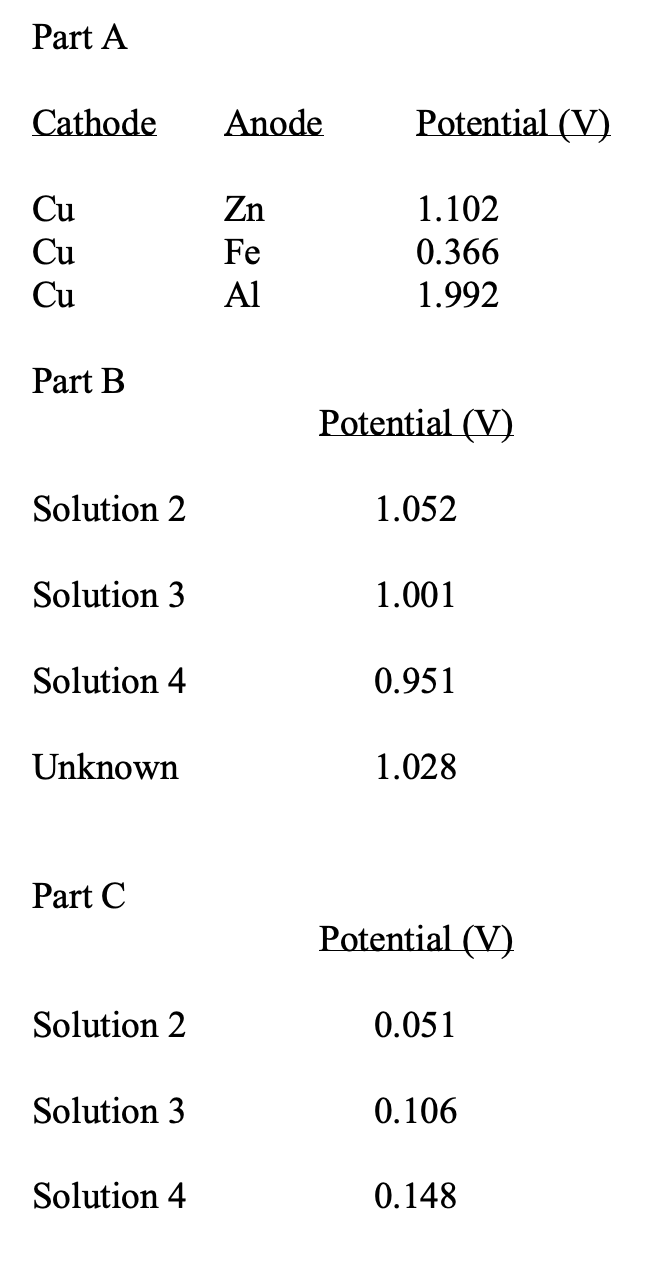
Transcribed Image Text:Part A
Cathode
Anode
Potential (V)
Cu
Zn
1.102
Cu
Fe
0.366
Cu
Al
1.992
Part B
Potential (V)
Solution 2
1.052
Solution 3
1.001
Solution 4
0.951
Unknown
1.028
Part C
Potential (V)
Solution 2
0.051
Solution 3
0.106
Solution 4
0.148
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,