• Part A The half-life for the radioactive decay of U - 238 is 4.5 billion years and is independent initial concentration. How long will it take for 16 % of the U - 238 atoms in a sample ofU - 238 to decay? Express your answer using two significant figures. t= 1.1x109 yr Previous Answers v Correct Correct answer shown. Your answer 11.323-10 = 1.132x10° yr was either rounded differently or used a different number of significant figures than required for this par • Part B If a sample of U– 238 initially contained 1.3x1018 atoms and was formed 6.1 billion years ago, how many U - 238 atoms does it contain today? Express your answer using two significant figures. Temp@es Symbols uado redo reses keyboard shortcuts help N = atoms
• Part A The half-life for the radioactive decay of U - 238 is 4.5 billion years and is independent initial concentration. How long will it take for 16 % of the U - 238 atoms in a sample ofU - 238 to decay? Express your answer using two significant figures. t= 1.1x109 yr Previous Answers v Correct Correct answer shown. Your answer 11.323-10 = 1.132x10° yr was either rounded differently or used a different number of significant figures than required for this par • Part B If a sample of U– 238 initially contained 1.3x1018 atoms and was formed 6.1 billion years ago, how many U - 238 atoms does it contain today? Express your answer using two significant figures. Temp@es Symbols uado redo reses keyboard shortcuts help N = atoms
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter12: Kinetics
Section: Chapter Questions
Problem 46E: Fluorine-18 is a radioactive isotope that decays by positron emission to form oxygen-18 with a...
Related questions
Question
I need help for part B
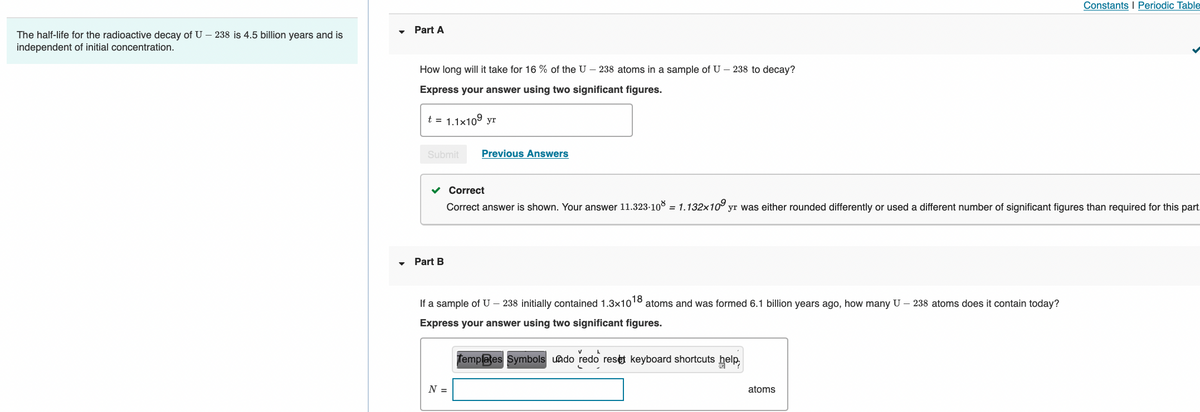
Transcribed Image Text:Constants I Periodic Table
Part A
The half-life for the radioactive decay of U – 238 is 4.5 billion years and is
independent of initial concentration.
How long will it take for 16 % of the U – 238 atoms in a sample of U – 238 to decay?
Express your answer using two significant figures.
t = 1.1x10° yr
Submit
Previous Answers
Correct
Correct answer is shown. Your answer 11.323-108 = 1.132x10° yr was either rounded differently or used a different number of significant figures than required for this part.
Part B
If a sample of U – 238 initially contained 1.3x10
18
atoms and was formed 6.1 billion years ago, how many U – 238 atoms does it contain today?
Express your answer using two significant figures.
Templates Symbols undo redo reset keyboard shortcuts help
N =
atoms
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning