Part A The Lewis structure for the chlorate ion is b :0: Calculate the formal charge on the chlorine (Cl) atom. Express your answer as an integer. • View Available Hint(s) formal charge on Cl = Part B Calculate the formal charge on each of the oxygen (O) atoms labeled a, b, and c in the following Lewis structure. b :0: Express your answers as integers separated by commas. • View Available Hint(s) formal charge on Oa, Op , Oc = :O: :0:
Part A The Lewis structure for the chlorate ion is b :0: Calculate the formal charge on the chlorine (Cl) atom. Express your answer as an integer. • View Available Hint(s) formal charge on Cl = Part B Calculate the formal charge on each of the oxygen (O) atoms labeled a, b, and c in the following Lewis structure. b :0: Express your answers as integers separated by commas. • View Available Hint(s) formal charge on Oa, Op , Oc = :O: :0:
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter6: Covalent Bonding
Section: Chapter Questions
Problem 45QRT
Related questions
Question
Please answer question 5 part A, B, and C
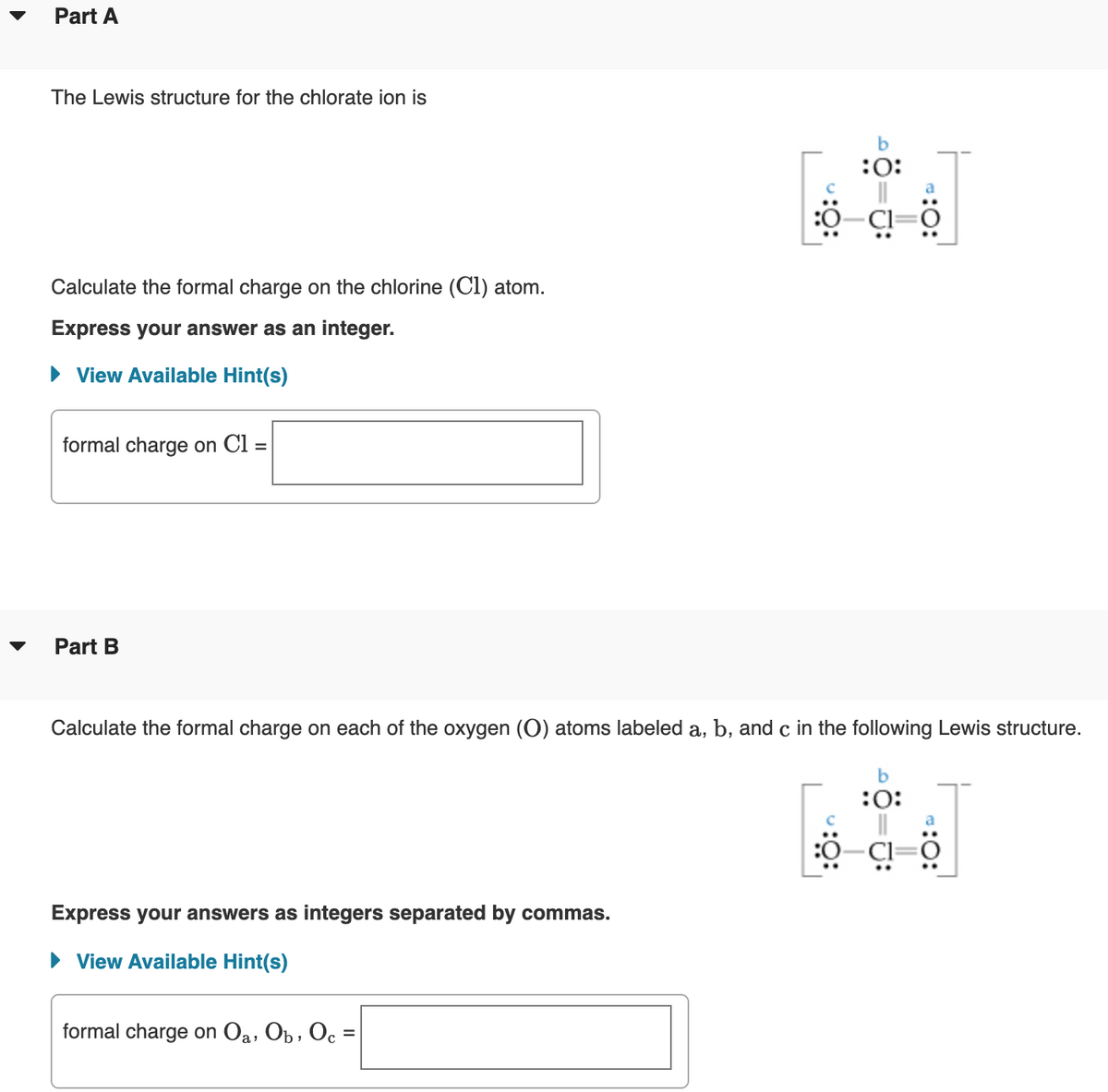
Transcribed Image Text:Part A
The Lewis structure for the chlorate ion is
b
:0:
Calculate the formal charge on the chlorine (Cl) atom.
Express your answer as an integer.
• View Available Hint(s)
formal charge on Cl =
Part B
▼
Calculate the formal charge on each of the oxygen (O) atoms labeled a, b, and c in the following Lewis structure.
:0:
Express your answers as integers separated by commas.
• View Available Hint(s)
formal charge on Oa, Ob , Oc =
:0:
![Part C
What are the formal charges on the sulfur (S), carbon (C), and nitrogen (N) atoms, respectively, in the resonance structure that contributes most to the stability of the thiocyanate ion,
SCN-?
The possible resonance structures for the thiocyanate ion, SCN¯, are
S=c=N]'
-c=N] [:s=c-Ñ]"
Structure A
Structure B
Structure C
Express your answers as integers separated by commas.
• View Available Hint(s)
formal charge on sulfur (S), carbon (C) and nitrogen (N) atoms =](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2Ff384261d-463c-4246-ae44-7c9978465400%2F765dc5b7-e497-41e2-8679-e83c832b24a2%2Fts9w8f_processed.png&w=3840&q=75)
Transcribed Image Text:Part C
What are the formal charges on the sulfur (S), carbon (C), and nitrogen (N) atoms, respectively, in the resonance structure that contributes most to the stability of the thiocyanate ion,
SCN-?
The possible resonance structures for the thiocyanate ion, SCN¯, are
S=c=N]'
-c=N] [:s=c-Ñ]"
Structure A
Structure B
Structure C
Express your answers as integers separated by commas.
• View Available Hint(s)
formal charge on sulfur (S), carbon (C) and nitrogen (N) atoms =
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning


Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning