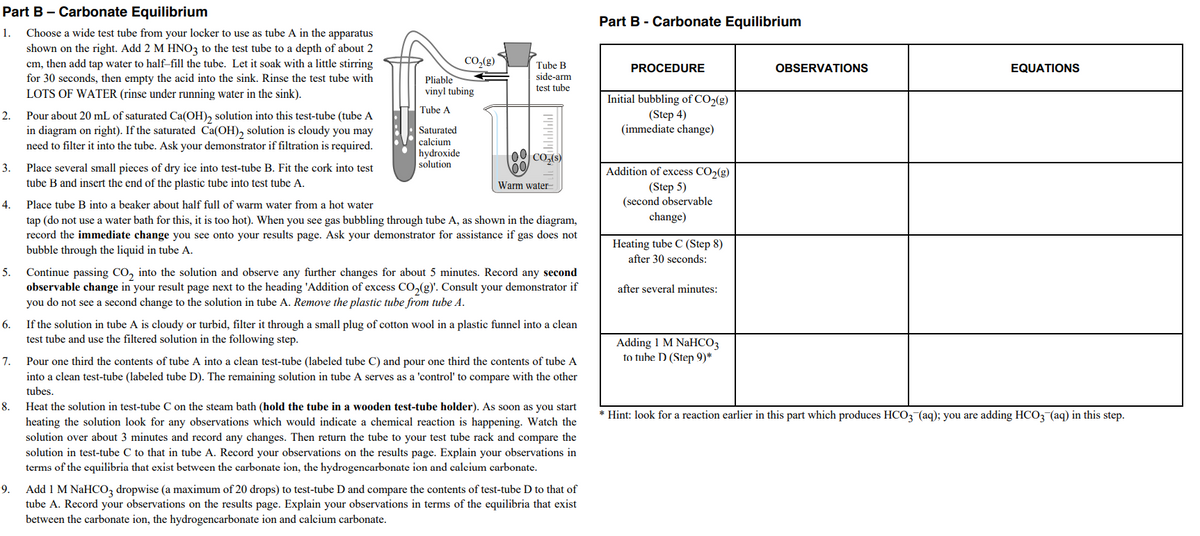
Part B- Carbonate Equilibrium Part B - Carbonate Equilibrium 1. Choose a wide test tube from your locker to use as tube A in the apparatus shown on the right. Add 2 M HNO3 to the test tube to a depth of about 2 cm, then add tap water to half-fill the tube. Let it soak with a little stirring for 30 seconds, then empty the acid into the sink. Rinse the test tube with LOTS OF WATER (rinse under running water in the sink). co.(g) Tube B side-arm test tube PROCEDURE OBSERVATIONS EQUATIONS Pliable vinyl tubing Initial bubbling of CO>(g) Tube A Pour about 20 mL of saturated Ca(OH), solution into this test-tube (tube A in diagram on right). If the saturated Ca(OH), solution is cloudy you may need to filter it into the tube. Ask your demonstrator if filtration is required. Saturated calcium hydroxide solution (Step 4) (immediate change) 00 co, 3. Place several small pieces of dry ice into test-tube B. Fit the cork into test tube B and insert the end of the plastic tube into test tube A. Addition of excess CO>(g) (Step 5) (second observable Warm water- 4. Place tube B into a beaker about half full of warm water from a hot water change) tap (do not use a water bath for this, it is too hot). When you see gas bubbling through tube A, as shown in the diagram, record the immediate change you see onto your results page. Ask your demonstrator for assistance if gas does not bubble through the liquid in tube A. Heating tube C (Step 8) after 30 seconds: Continue passing CO, into the solution and observe any further changes for about 5 minutes. Record any second observable change in your result page next to the heading 'Addition of excess CO,(g). Consult your demonstrator if you do not see a second change to the solution in tube A. Remove the plastic tube from tube A. after several minutes: 5. If the solution in tube A is cloudy or turbid, filter it through a small plug of cotton wool in a plastic funnel into a clean test tube and use the filtered solution in the following step. Adding I M NaHCO3 to tuhe D (Step 9)* 7. Pour one third the contents of tube A into a clean test-tube (labeled tube C) and pour one third the contents of tube A into a clean test-tube (labeled tube D). The remaining solution in tube A serves as a 'control' to compare with the other tubes. Heat the solution in test-tube C on the steam bath (hold the tube in a wooden test-tube holder). As soon as you start heating the solution look for any observations which would indicate a chemical reaction is happening. Watch the solution over about 3 minutes and record any changes. Then return the tube to your test tube rack and compare the solution in test-tube C to that in tube A. Record your observations on the results page. Explain your observations in terms of the equilibria that exist between the carbonate ion, the hydrogencarbonate ion and calcium carbonate. • Hint: look for a reaction earlier in this part which produces HCO3¬(aq); you are adding HCO; (aq) in this step. . Add 1 M NaHCO3 dropwise (a maximum of 20 drops) to test-tube D and compare the contents of test-tube D to that of tube A. Record your observations on the results page. Explain your observations in terms of the equilibria that exist between the carbonate ion, the hydrogencarbonate ion and calcium carbonate.
Ionic Equilibrium
Chemical equilibrium and ionic equilibrium are two major concepts in chemistry. Ionic equilibrium deals with the equilibrium involved in an ionization process while chemical equilibrium deals with the equilibrium during a chemical change. Ionic equilibrium is established between the ions and unionized species in a system. Understanding the concept of ionic equilibrium is very important to answer the questions related to certain chemical reactions in chemistry.
Arrhenius Acid
Arrhenius acid act as a good electrolyte as it dissociates to its respective ions in the aqueous solutions. Keeping it similar to the general acid properties, Arrhenius acid also neutralizes bases and turns litmus paper into red.
Bronsted Lowry Base In Inorganic Chemistry
Bronsted-Lowry base in inorganic chemistry is any chemical substance that can accept a proton from the other chemical substance it is reacting with.
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images









