Use the following information to answer the next question. A sample solution contains two unknown ions. A student did a series of tests on the solution, in the order listed below. If a precipitate formed at any stage, the mixture was filtered and further tests were done on the filtrate. The results are as follows. First test: solution is a blue-green colour NaBr(aq) is added and a precipitate forms flame colour is violet Second test: Third test: Fourth test: Naclo,(ag) is added and no precipitate forms Two possible ions that the solution contains are O a. copper(I) and cesium O b. lead(II) and potassium O c. copper(I) and potassium O d. lead(II) and rubidium
Use the following information to answer the next question. A sample solution contains two unknown ions. A student did a series of tests on the solution, in the order listed below. If a precipitate formed at any stage, the mixture was filtered and further tests were done on the filtrate. The results are as follows. First test: solution is a blue-green colour NaBr(aq) is added and a precipitate forms flame colour is violet Second test: Third test: Fourth test: Naclo,(ag) is added and no precipitate forms Two possible ions that the solution contains are O a. copper(I) and cesium O b. lead(II) and potassium O c. copper(I) and potassium O d. lead(II) and rubidium
Chapter1: Chemistry: An Introduction
Section: Chapter Questions
Problem 11A
Related questions
Question
Can you please answer these and give me an explanation.
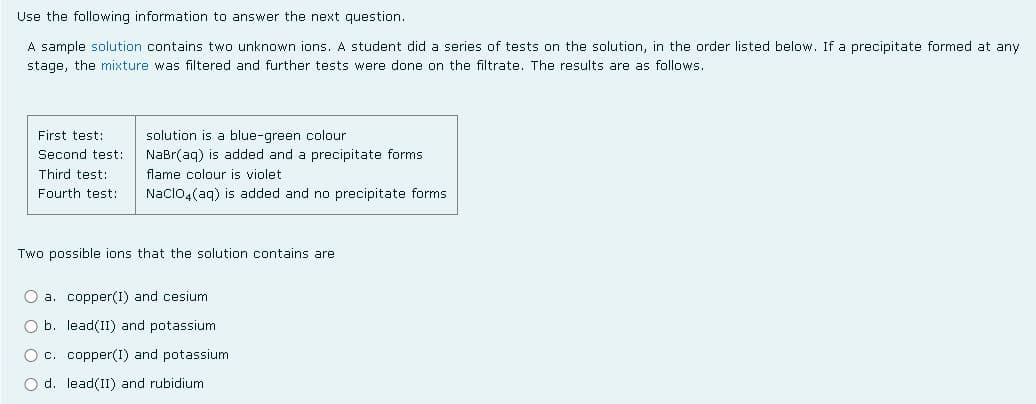
Transcribed Image Text:Use the following information to answer the next question.
A sample solution contains two unknown ions. A student did a series of tests on the solution, in the order listed below. If a precipitate formed at any
stage, the mixture was filtered and further tests were done on the filtrate. The results are as follows.
First test:
solution is a blue-green colour
NaBr(aq) is added and a precipitate forms
flame colour is violet
Second test:
Third test:
Fourth test:
Nacloa(ag) is added and no precipitate forms
Two possible ions that the solution contains are
O a. copper(I) and cesium
O b. lead(II) and potassium
O c. copper(I) and potassium
O d. lead(II) and rubidium

Transcribed Image Text:For each of the following observations, indicate whether it is an example of a quantitative or qualitative observation.
Qualitative or Quantitative?
Observation
quantitative
Bromothymol blue indicator turns yellow when it is added to a solution.
Qualitative or Quantitative?
Observation
When the chemical reaction was complete, 3.45 g of precipitate was produced.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

World of Chemistry
Chemistry
ISBN:
9780618562763
Author:
Steven S. Zumdahl
Publisher:
Houghton Mifflin College Div

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

World of Chemistry
Chemistry
ISBN:
9780618562763
Author:
Steven S. Zumdahl
Publisher:
Houghton Mifflin College Div

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning