Part B Write the chemical equation for the reaction of propylamine with water. The format should resemble the general reaction scheme given in the introduction of this problem. Express your answer as a chemical equation including condensed structural formula.
Part B Write the chemical equation for the reaction of propylamine with water. The format should resemble the general reaction scheme given in the introduction of this problem. Express your answer as a chemical equation including condensed structural formula.
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter9: Liquids And Solids
Section: Chapter Questions
Problem 65QAP: Chloroform, CHCl3, has a normal boiling point of 61C. Its vapor pressure at 43C is 0.526 atm. What...
Related questions
Question
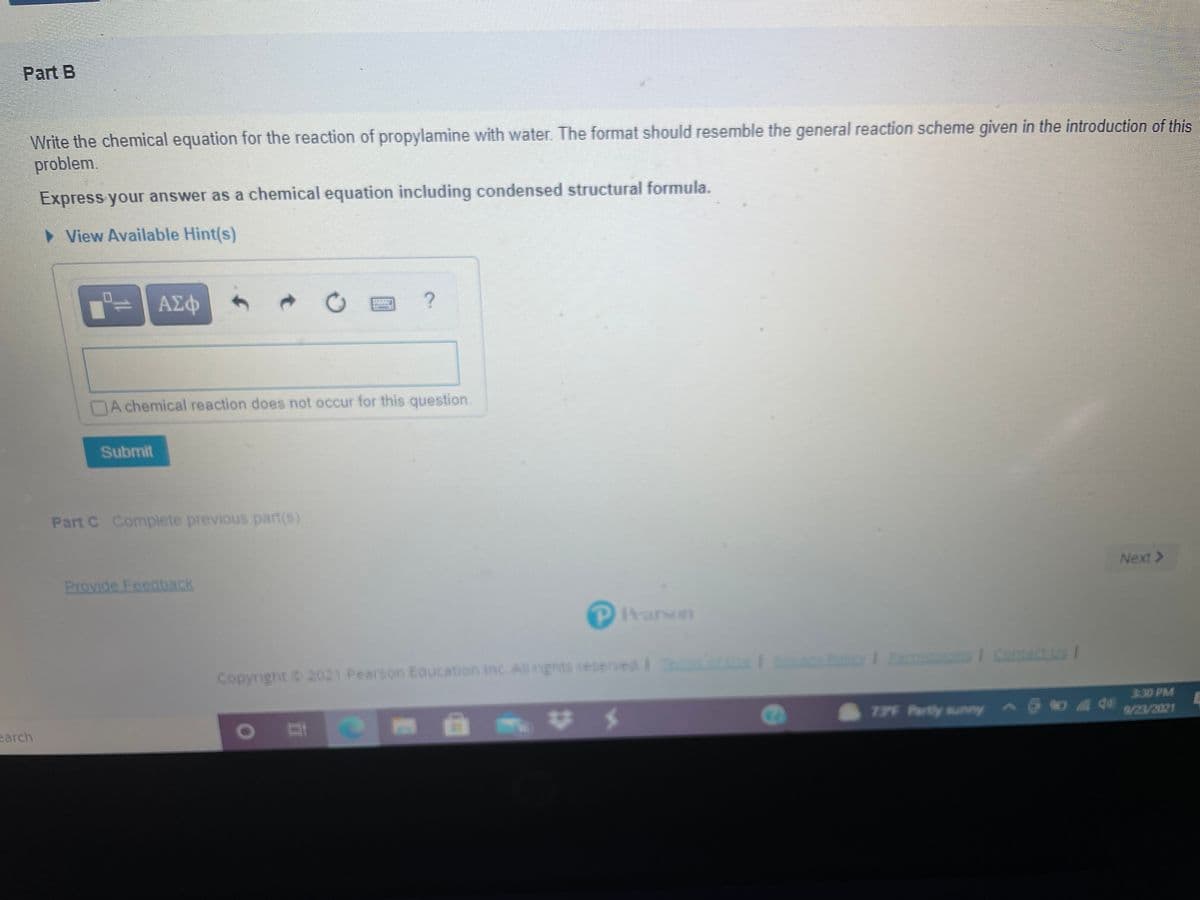
Transcribed Image Text:Part B
Write the chemical equation for the reaction of propylamine with water. The format should resemble the general reaction scheme given in the introduction of this
problem.
Express your answer as a chemical equation including condensed structural formula.
> View Available Hint(s)
ΑΣφ
A chemical reaction does not occur for this question.
Submit
Part C Complete previous part(s)
Provide Feecback
Next>
Pharson
Copyright 2021 Pearson Education inc All nghts resened.
पचदद प्र ७॥
330 PM
> 耳。
77 Partly sunny A @ o a Q0
earch
9/23/2021
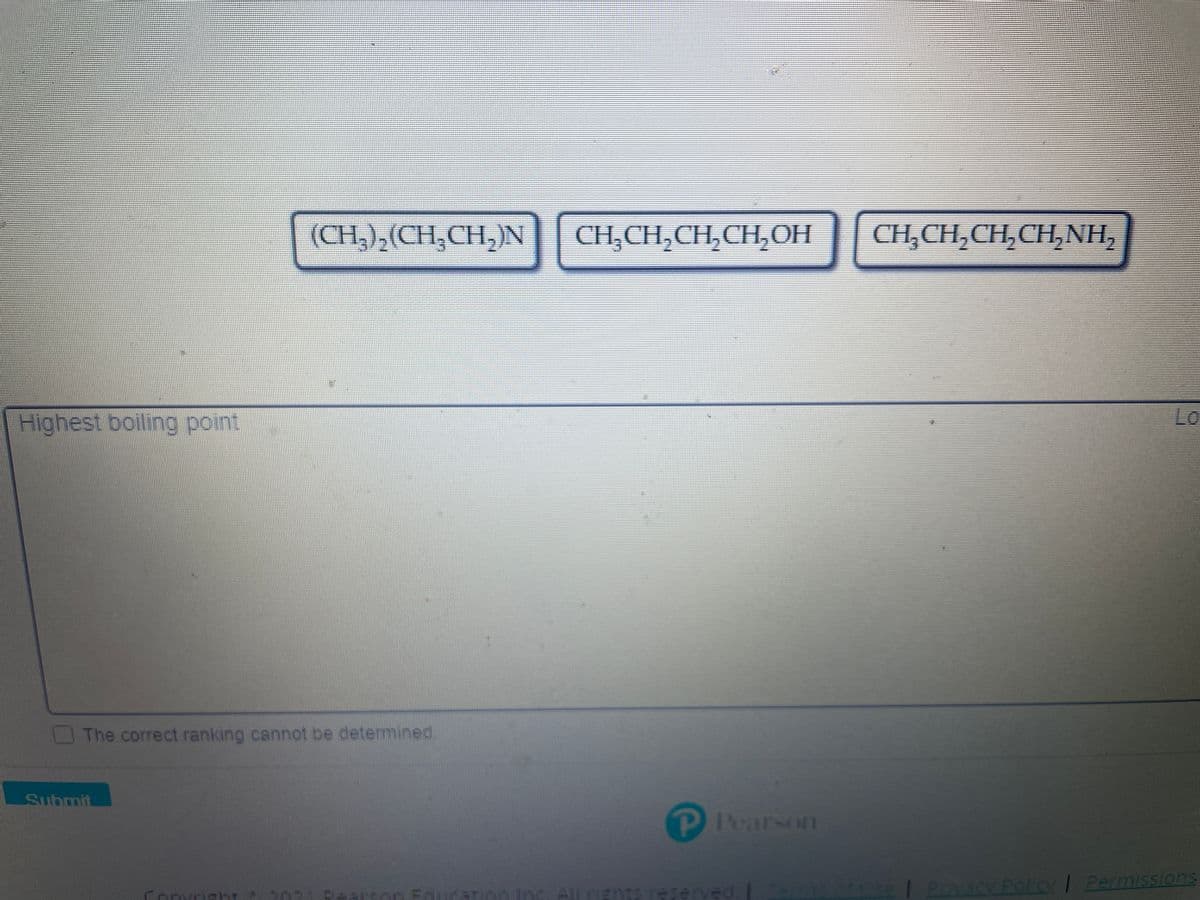
Transcribed Image Text:(CH,),(CH,CH,)N
CH,CH,CH,CH,OH
CH,CH,CH,CH,NH,
Highest boiling point
Lo
The correct ranking cannot be determined.
Submit
PRearson
Allngnts reserved
e PoA Polty I Permissions
Cony
hr
Fdi
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning