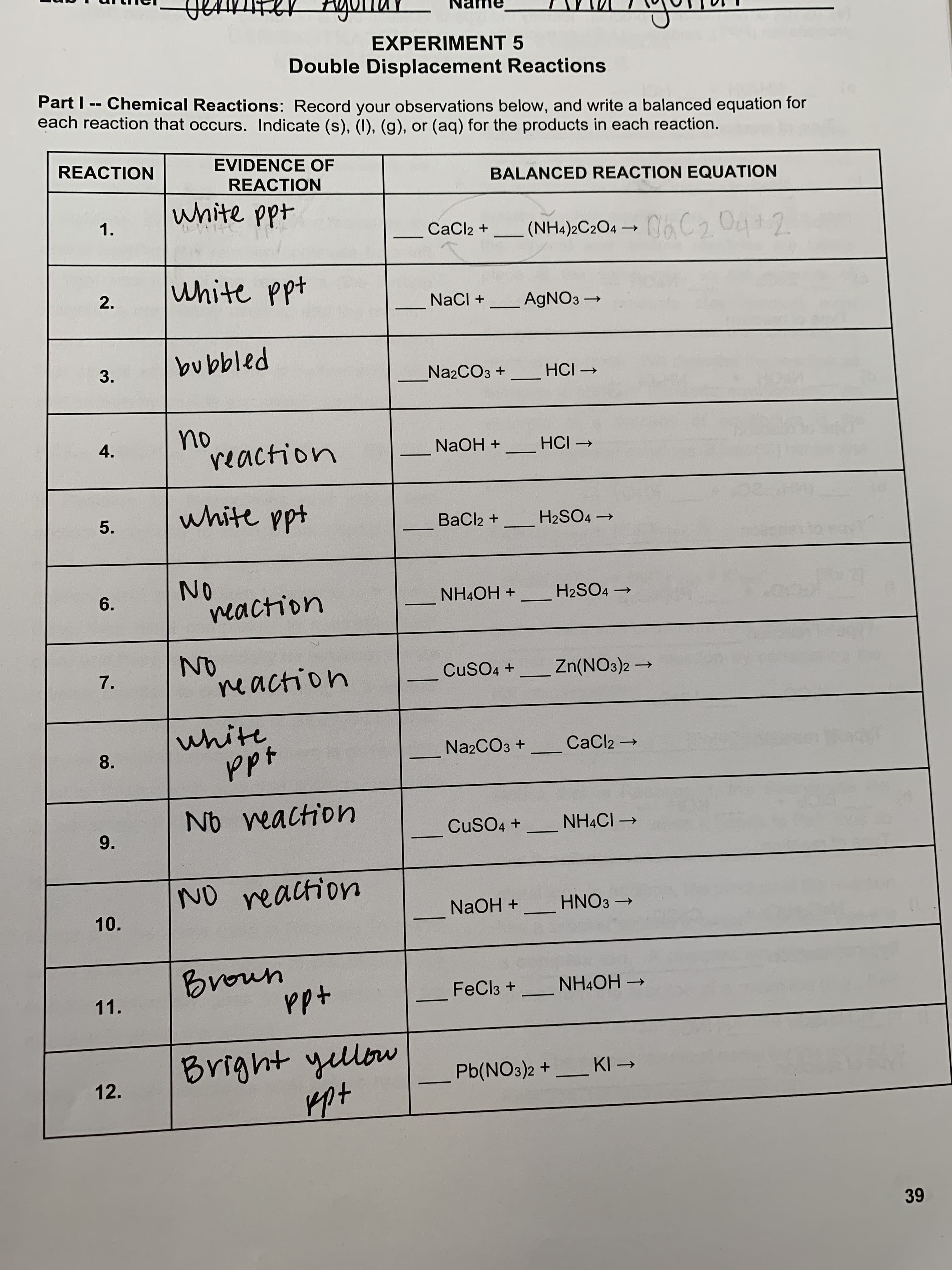
Part I -- Chemical Reactions: Record your observations below, and write a balanced equation for each reaction that occurs. Indicate (s), (I), (g), or (aq) for the products in each reaction. EVIDENCE OF REACTION BALANCED REACTION EQUATION REACTION white ppt CaCl2 + (NH4)2C2O4 → C C2 O4+2 1. white ppt NaCI + -AGNO3 → bubbled HCI → N22CO3 + no reaction NaOH + HCI → 4. white ppt BaCl2 + H2SO4 → 5. 2. 3.
Part I -- Chemical Reactions: Record your observations below, and write a balanced equation for each reaction that occurs. Indicate (s), (I), (g), or (aq) for the products in each reaction. EVIDENCE OF REACTION BALANCED REACTION EQUATION REACTION white ppt CaCl2 + (NH4)2C2O4 → C C2 O4+2 1. white ppt NaCI + -AGNO3 → bubbled HCI → N22CO3 + no reaction NaOH + HCI → 4. white ppt BaCl2 + H2SO4 → 5. 2. 3.
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter7: Reactions In Aqueous Solutions
Section: Chapter Questions
Problem 13CR: What is a combustion reaction? Are combustion reactions a unique type of reaction, or are they a...
Related questions
Question
Transcribed Image Text:39
3.
EXPERIMENT 5
Double Displacement Reactions
Part I -- Chemical Reactions: Record your observations below, and write a balanced equation for
each reaction that occurs. Indicate (s), (1), (g), or (ag) for the products in each reaction.
EVIDENCE OF
REACTION
BALANCED REACTION EQUATION
REACTION
white ppt
C2 O4+2
1.
CaCl2 +
(NH4)2C2O4 →
+dd
ite
2.
NaCI +
_AGNO3
Pa1qana
N22CO3 +_ HCI →
no
reaction
4.
NaOH +
HCI →
BaCl2 +
H2SO4 →
5.
dd
O.
reaction
H2SO4
+ HO*HN
6.
CuSO4 + Zn(NO3)2
→
ne action
7.
N22CO3 +
CaCl2 →
8.
No veaction
CuSO4 +
NHẠCI -
9.
NO reaction
+ HH.
HNO3 →
10.
umarg
+dd
-
FeCl3 + NH4OH
11.
moh tubug
Pb(NO3)2 + _ KI →
12.

Transcribed Image Text:Part II -- Reacțion Equations: Write a balanced equation for each of the following reactions. Indicate
(s), (I), (g), or (aq) for each product. Identify the type of reaction that is occurring: No Reaction (NR),
precipitation (PPT), acid/base (A/B), or evolution of gas (GAS).
+ HO*HN
+ IƆH
Type of reaction
Na2S +
CuSO4
→
Type of reaction
ZnCl2 +
一
+ HOBN
Type of reaction
(p
+ 19*HN
heat
+ HO
Type of reaction
NO
(NH4)2SO4 +
KNO3
Type of reaction
K2CrO4 +
Pb(NO3)2
Type of reaction
ACHON
K2CO3 +
+ EONH
Type of reaction
BiCl3 +
HOH
Type of reaction
1)
Type of reaction
NaC2H3O2 + COSO4 →
N22SO3 +
+EONH
Type of reaction
40
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning


General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning