Part II. Problem Solving (20 pts) Instruction: Solve the colligative property questions. Refer below the values needed in the equation. Show your solution. Solvent Freezing Kf (°C/m) Kb(*C/m) Boiling point(°C) Point(°C) Water 100 1.858 0.521 Acetic acid 16.60 118.5 3.59 3.08 1. At what temperature will a 2.5 molar of salt in Acetic acid (vinegar) boil?
Part II. Problem Solving (20 pts) Instruction: Solve the colligative property questions. Refer below the values needed in the equation. Show your solution. Solvent Freezing Kf (°C/m) Kb(*C/m) Boiling point(°C) Point(°C) Water 100 1.858 0.521 Acetic acid 16.60 118.5 3.59 3.08 1. At what temperature will a 2.5 molar of salt in Acetic acid (vinegar) boil?
Chemical Principles in the Laboratory
11th Edition
ISBN:9781305264434
Author:Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Chapter3: Resolution Of Matter Into Pure Substances, Ii. Fractional Crystallization
Section: Chapter Questions
Problem 1ASA: Using Figure 3.1, determine a. the number of grams of CuSO45H2O that will dissolve in 100g of H2O at...
Related questions
Question
note: the question number 1 is 2.5 molal not molar.
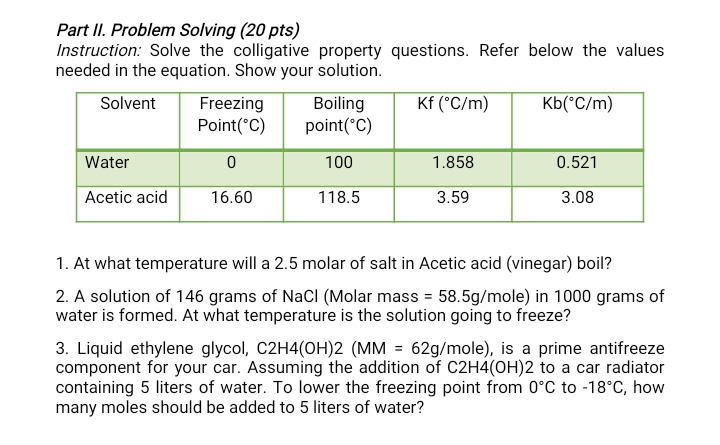
Transcribed Image Text:Part II. Problem Solving (20 pts)
Instruction: Solve the colligative property questions. Refer below the values
needed in the equation. Show your solution.
Solvent
Freezing
Boiling
Kf (°C/m)
Kb(*C/m)
Point(°C)
point(°C)
Water
100
1.858
0.521
Acetic acid
16.60
118.5
3.59
3.08
1. At what temperature will a 2.5 molar of salt in Acetic acid (vinegar) boil?
2. A solution of 146 grams of NaCI (Molar mass = 58.5g/mole) in 1000 grams of
water is formed. At what temperature is the solution going to freeze?
3. Liquid ethylene glycol, C2H4(OH)2 (MM = 62g/mole), is a prime antifreeze
component for your car. Assuming the addition of C2H4(OH)2 to a car radiator
containing 5 liters of water. To lower the freezing point from 0°C to -18°C, how
many moles should be added to 5 liters of water?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning