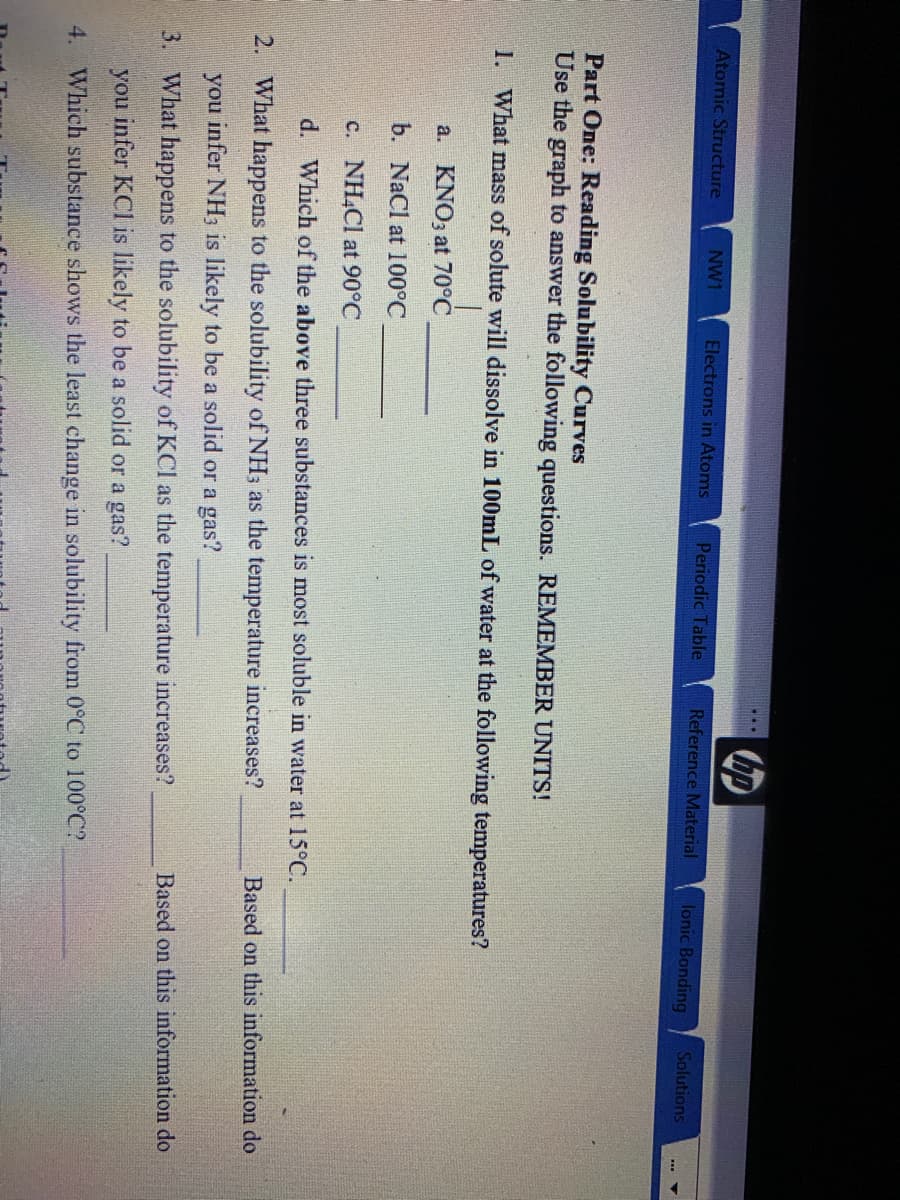
Part One: Reading Solubility Curves Use the graph to answer the following questions. REMEMBER UNITS! 1. What mass of solute will dissolve in 100mL of water at the following temperatures? a. KNO3 at 70°C b. NaCl at 100°C c. NH4CI at 90°C d. Which of the above three substances is most soluble in water at 15°C. 2. What happens to the solubility of NH; as the temperature increases? Based on this information do you infer NH3 is likely to be a solid or a gas? 3. What happens to the solubility of KCl as the temperature increases? Based on this information do you infer KCl is likely to be a solid or a gas? 4. Which substance shows the least change in solubility from 0°C to 100°C?
Part One: Reading Solubility Curves Use the graph to answer the following questions. REMEMBER UNITS! 1. What mass of solute will dissolve in 100mL of water at the following temperatures? a. KNO3 at 70°C b. NaCl at 100°C c. NH4CI at 90°C d. Which of the above three substances is most soluble in water at 15°C. 2. What happens to the solubility of NH; as the temperature increases? Based on this information do you infer NH3 is likely to be a solid or a gas? 3. What happens to the solubility of KCl as the temperature increases? Based on this information do you infer KCl is likely to be a solid or a gas? 4. Which substance shows the least change in solubility from 0°C to 100°C?
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter12: Solutions
Section: Chapter Questions
Problem 12.98QE
Related questions
Question
100%
Transcribed Image Text:Atomic Structure
NW1
Electrons in Atoms
Periodic Table
Reference Material
Solutions
lonic Bonding
Part One: Reading Solubility Curves
Use the graph to answer the following questions. REMEMBER UNITS!
1. What mass of solute will dissolve in 100ML of water at the following temperatures?
a. KNO3 at 70°C
b. NaCl at 100°C
c. NH,Cl at 90°C
d. Which of the above three substances is most soluble in water at 15°C.
2. What happens to the solubility of NH3 as the temperature increases?
Based on this information do
you infer NH3 is likely to be a solid or a gas?
Based on this information do
3. What happens to the solubility of KCl as the temperature increases?
you infer KCl is likely to be a solid or a gas?
4. Which substance shows the least change in solubility from 0°C to 100°C?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning
