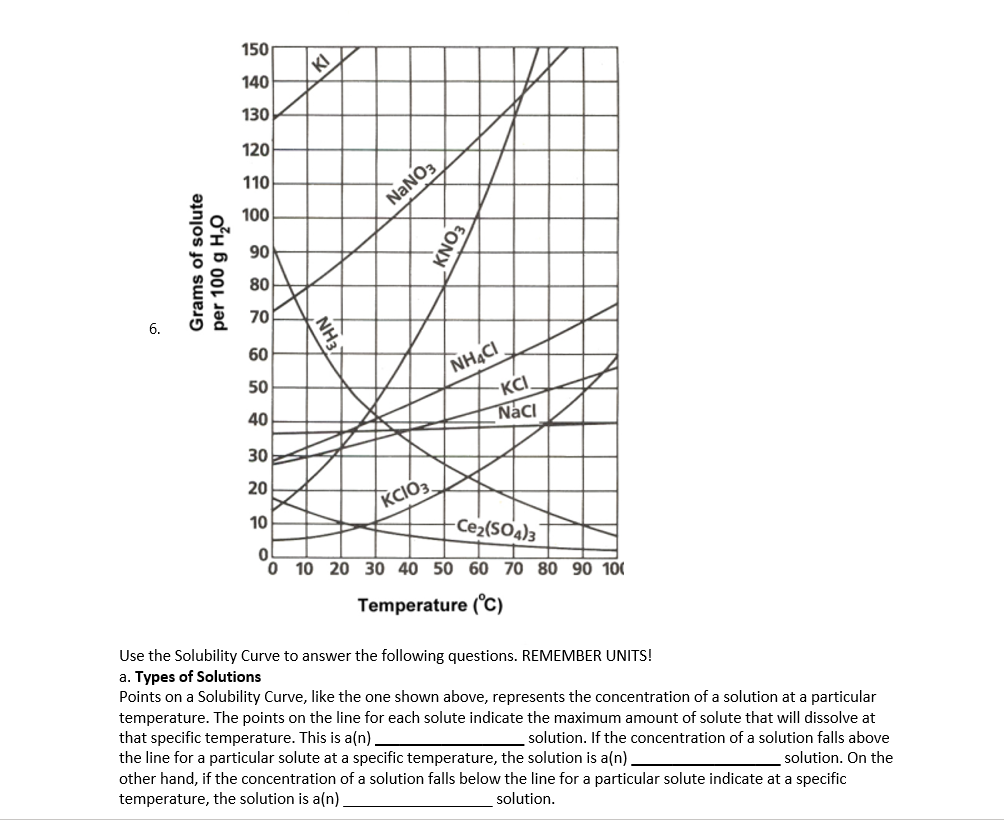
Use the Solubility Curve to answer the following questions. REMEMBER UNITS! a. Types of Solutions Points on a Solubility Curve, like the one shown above, represents the concentration of a solution at a particular temperature. The points on the line for each solute indicate the maximum amount of solute that will dissolve at that specific temperature. This is a(n). the line for a particular solute at a specific temperature, the solution is a(n). other hand, if the concentration of a solution falls below the line for a particular solute indicate at a specific temperature, the solution is a(n). solution. If the concentration of a solution falls above solution. On the solution.
Use the Solubility Curve to answer the following questions. REMEMBER UNITS! a. Types of Solutions Points on a Solubility Curve, like the one shown above, represents the concentration of a solution at a particular temperature. The points on the line for each solute indicate the maximum amount of solute that will dissolve at that specific temperature. This is a(n). the line for a particular solute at a specific temperature, the solution is a(n). other hand, if the concentration of a solution falls below the line for a particular solute indicate at a specific temperature, the solution is a(n). solution. If the concentration of a solution falls above solution. On the solution.
General, Organic, and Biological Chemistry
7th Edition
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:H. Stephen Stoker
Chapter8: Solutions
Section: Chapter Questions
Problem 8.7EP: Classify each of the following solutions as saturated, unsaturated, or supersaturated based on the...
Related questions
Question
Transcribed Image Text:150
140
130
120
110
100
NANO3
90
80A
70
60
NH4CI
KCI.
Naci
50
40
30
20
KCIO3
10
Ce2(SO4)3
O 10 20 30 40 50 60 70 80 90 100
Temperature (°C)
Use the Solubility Curve to answer the following questions. REMEMBER UNITS!
a. Types of Solutions
Points on a Solubility Curve, like the one shown above, represents the concentration of a solution at a particular
temperature. The points on the line for each solute indicate the maximum amount of solute that will dissolve at
that specific temperature. This is a(n).
the line for a particular solute at a specific temperature, the solution is a(n).
other hand, if the concentration of a solution falls below the line for a particular solute indicate at a specific
temperature, the solution is a(n)
solution. If the concentration of a solution falls above
solution. On the
solution.
Grams of solute
per 100 g H,0
NH3)
EON
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning
