PERCENTAGE ERROR Name Percentage error is a way for scientists to express how far off a laboratory value is from the commonly accepted value. The formula is: Accepted Value - Experimental Value % error x 100 Accepted Value absolute value Determine the percentage error in the following problems. 1. Experimental Value 1.24g 30 1,2d 98 Accepted Value = 1.30 g Answer: 20 2. Experimental Value 1.24x 102 g Accepted Value 9.98 x 10 g Answer: 3. Experimental Value 252 mL Accepted Value 225 mL Answer: 4. Experimental Value 22.2L Accepted Value = 22.4 L 0M Answer: 5. Experimental Value 125.2 mg Accepted Value = 124.8 mg Answer: @instructional Fair, In 11 Chemlstry IF8766
PERCENTAGE ERROR Name Percentage error is a way for scientists to express how far off a laboratory value is from the commonly accepted value. The formula is: Accepted Value - Experimental Value % error x 100 Accepted Value absolute value Determine the percentage error in the following problems. 1. Experimental Value 1.24g 30 1,2d 98 Accepted Value = 1.30 g Answer: 20 2. Experimental Value 1.24x 102 g Accepted Value 9.98 x 10 g Answer: 3. Experimental Value 252 mL Accepted Value 225 mL Answer: 4. Experimental Value 22.2L Accepted Value = 22.4 L 0M Answer: 5. Experimental Value 125.2 mg Accepted Value = 124.8 mg Answer: @instructional Fair, In 11 Chemlstry IF8766
Principles of Instrumental Analysis
7th Edition
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
ChapterA1: Evaluation Of Analytical Data
Section: Chapter Questions
Problem A1.1QAP
Related questions
Question
100%
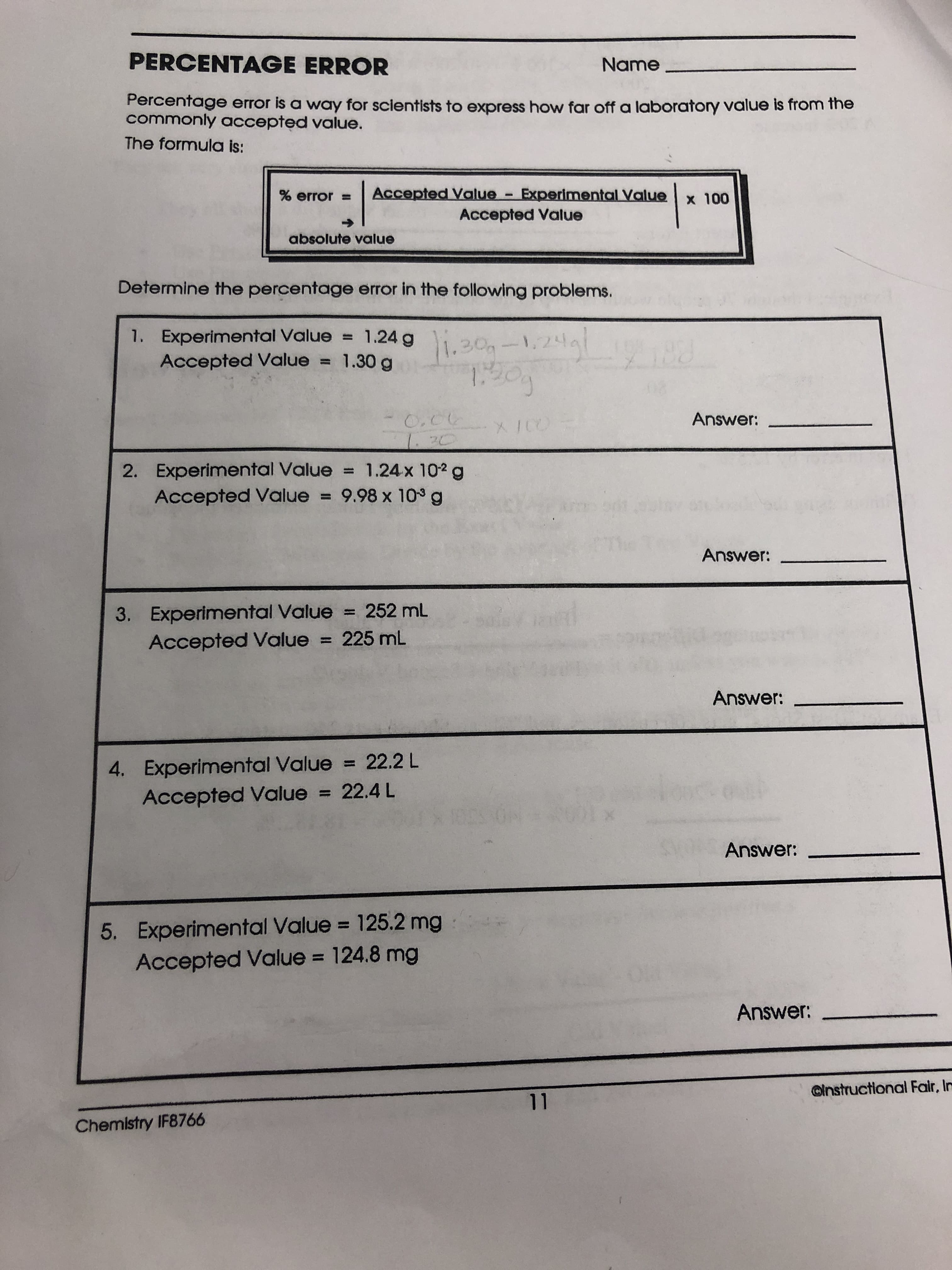
Transcribed Image Text:PERCENTAGE ERROR
Name
Percentage error is a way for scientists to express how far off a laboratory value is from the
commonly accepted value.
The formula is:
Accepted Value - Experimental Value
% error
x 100
Accepted Value
absolute value
Determine the percentage error in the following problems.
1. Experimental Value 1.24g 30
1,2d
98
Accepted Value = 1.30 g
Answer:
20
2. Experimental Value 1.24x 102 g
Accepted Value 9.98 x 10 g
Answer:
3. Experimental Value 252 mL
Accepted Value 225 mL
Answer:
4. Experimental Value 22.2L
Accepted Value = 22.4 L
0M
Answer:
5. Experimental Value 125.2 mg
Accepted Value = 124.8 mg
Answer:
@instructional Fair, In
11
Chemlstry IF8766
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning
