Periodic Table Matching - Part B Periodic Table Matching - Part C Argon (Ar.) A. Atomic mass is more than Li Tellurium (Te) A. Is likely a gas at room temp, but less than B lodine (1) Fluorine (F) B. Similar reactivity to Cr B. Similar reactivity to Cl C.Located in Period 2 Francium (Fr) C. Properties of both metals and nonmetals Beryllium (Be) Oxygen (O) D. One of the noble gases Tungsten (W) D. Atomic number is 28 Radium (Ra) E. Has 88 protons Nickel (Ni) E. Located in Group 1 ng (A & B), find the definition that best matches each term Then type the letter of that Definition into the YELLOW box
Periodic Table Matching - Part B Periodic Table Matching - Part C Argon (Ar.) A. Atomic mass is more than Li Tellurium (Te) A. Is likely a gas at room temp, but less than B lodine (1) Fluorine (F) B. Similar reactivity to Cr B. Similar reactivity to Cl C.Located in Period 2 Francium (Fr) C. Properties of both metals and nonmetals Beryllium (Be) Oxygen (O) D. One of the noble gases Tungsten (W) D. Atomic number is 28 Radium (Ra) E. Has 88 protons Nickel (Ni) E. Located in Group 1 ng (A & B), find the definition that best matches each term Then type the letter of that Definition into the YELLOW box
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter1: The Nature Of Chemistry
Section1.13: The Periodic Table
Problem 1.9E
Related questions
Question
match the right letter with the right answers.
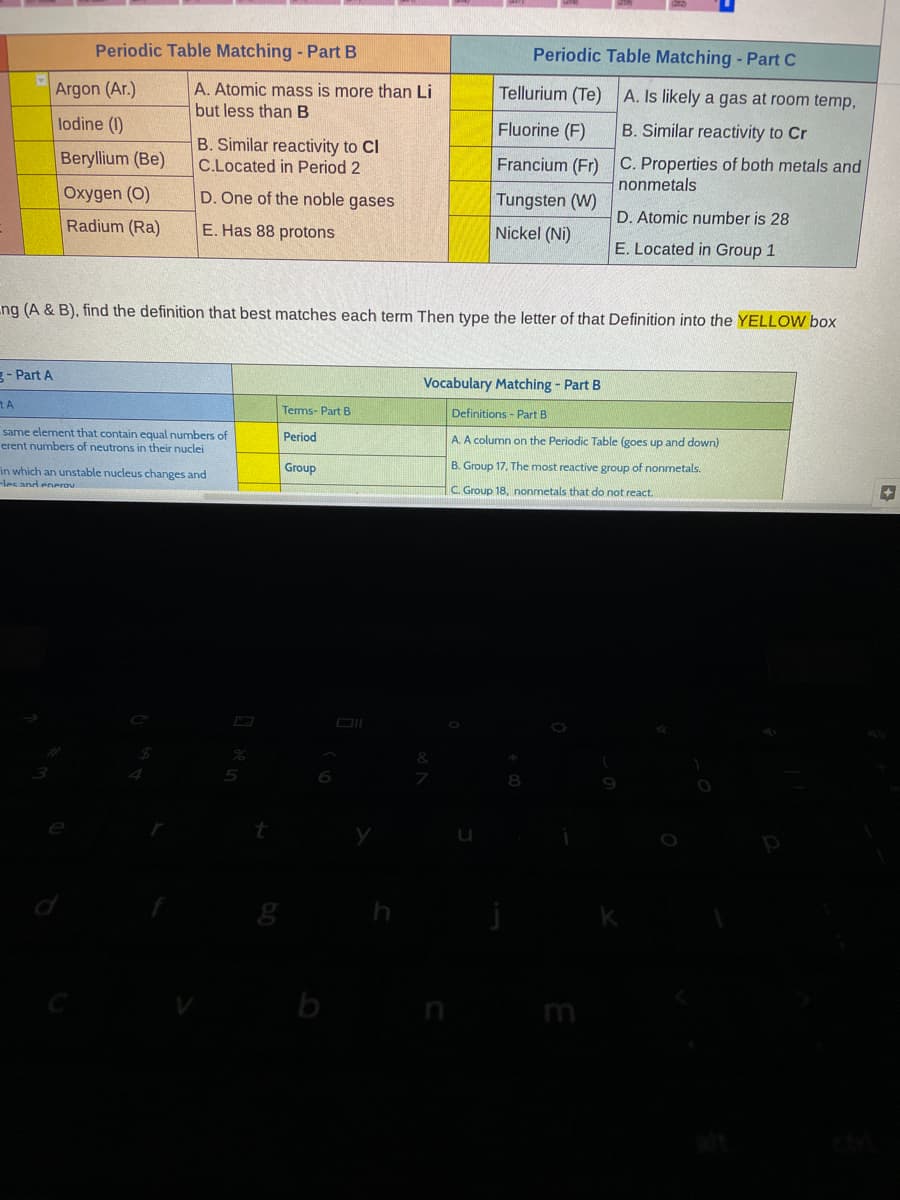
Transcribed Image Text:Periodic Table Matching - Part B
Periodic Table Matching - Part C
Argon (Ar.)
A. Atomic mass is more than Li
Tellurium (Te)
A. Is likely a gas at room temp,
but less than B
lodine (1)
Fluorine (F)
B. Similar reactivity to Cr
Beryllium (Be)
B. Similar reactivity to Cl
C.Located in Period 2
Francium (Fr) C. Properties of both metals and
nonmetals
Oxygen (O)
D. One of the noble gases
Tungsten (W)
Radium (Ra)
E. Has 88 protons
Nickel (Ni)
D. Atomic number is 28
E. Located in Group 1
ng (A & B), find the definition that best matches each term Then type the letter of that Definition into the YELLOW box
g- Part A
Vocabulary Matching - Part B
Terms- Part B
Definitions - Part B
same element that contain equal numbers of
erent numbers of neutrons in their nuclei
Period
A. A column on the Periodic Table (goes up and down)
B. Group 17, The most reactive group of nonmetals.
in which an unstable nucleus changes and
-les and enerov
Group
C. Group 18, nonmetals that do not react.
m
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 5 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

