Periodic Table Polyatomic lons List Conversion Factors Solubility Rules Consider the following reaction: 2 HCI + CaCO3 CaCl2 + H20 + CO2 How many grams of calcium chloride can be produced if you begin with 8.36 mL of 1.85 M HCI a 6.910 grams of calcium carbonate? g CaCl2 Identify the limiting reactant? O CaCO3 О НI Show your work: Graph to add drawings to:
Periodic Table Polyatomic lons List Conversion Factors Solubility Rules Consider the following reaction: 2 HCI + CaCO3 CaCl2 + H20 + CO2 How many grams of calcium chloride can be produced if you begin with 8.36 mL of 1.85 M HCI a 6.910 grams of calcium carbonate? g CaCl2 Identify the limiting reactant? O CaCO3 О НI Show your work: Graph to add drawings to:
ChapterU4: Toxins: Stoichiometry, Solution Chemistry, And Acids And Bases
Section: Chapter Questions
Problem 20STP
Related questions
Question
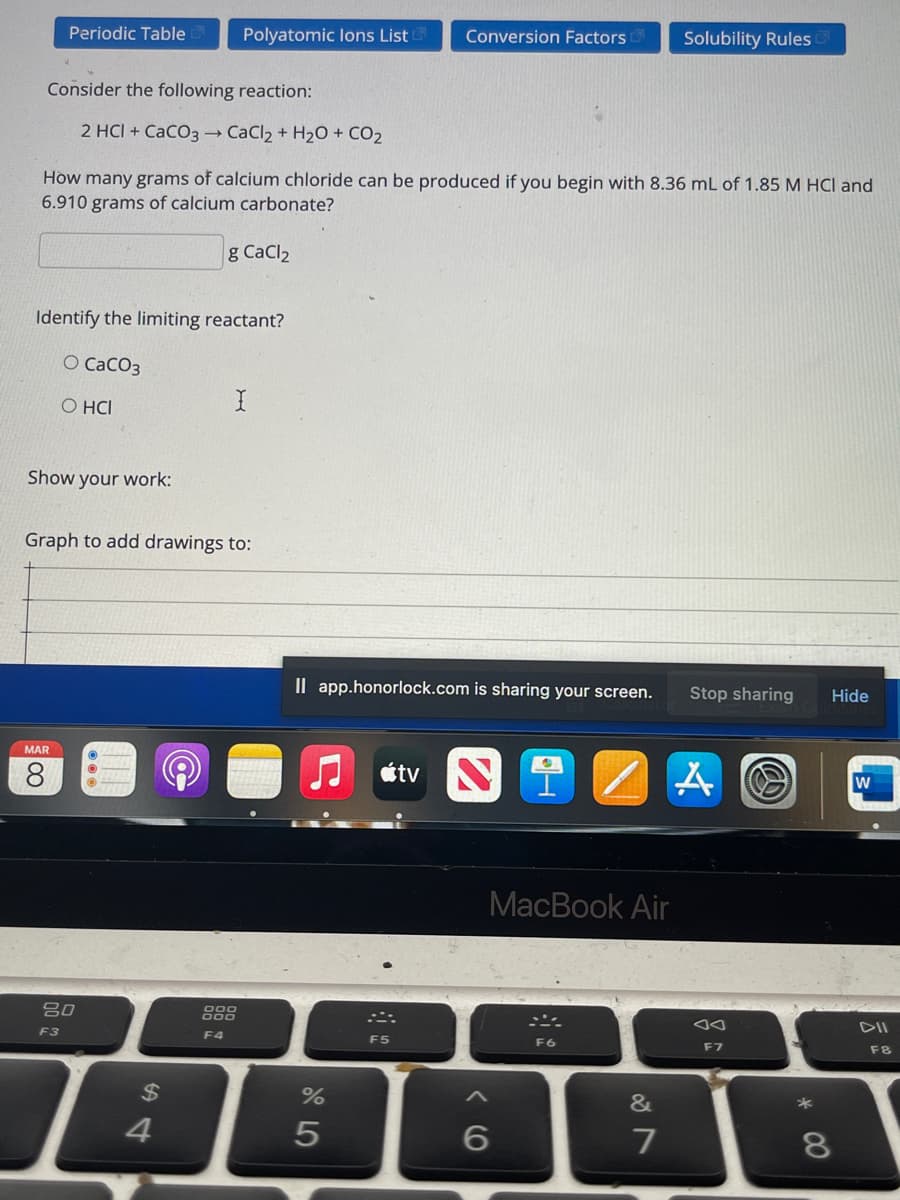
Transcribed Image Text:Periodic Table
Polyatomic lons List
Conversion Factors
Solubility Rules
Consider the following reaction:
2 HCI + CaCO3 CaCl2 + H20+ CO2
How many grams of calcium chloride can be produced if you begin with 8.36 mL of 1.85 M HCI and
6.910 grams of calcium carbonate?
g CaCl2
Identify the limiting reactant?
O CaCO3
O HCI
Show your work:
Graph to add drawings to:
Il app.honorlock.com is sharing your screen.
Stop sharing
Hide
MAR
8.
étv NT
w
МacBook Air
80
F3
F4
DII
F5
F6
F7
F8
$4
%
&
4.
7
8.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning