PhC=CH + NH3 = Phenylacetylene Ammonia Table 23.2 Acid Strengths, pK, of the Conjugate Acids of Selected Amines Amine Structure pk, of Conjugate Acld Ammonia NH, 9.26 Primary Amines Methylamine CH,NH, 10.64 Ethylamine CH,CH,NH, 10.81 Cyclohexylamine CH,NH, 10.66 Secondary Amines Dimethylamlne (CH),NH 10.73 Diethylamine (CH,CH,),NH 10.98 Tertiary Amines Trimethylamine (CH,),N 9.81 Triethylamine (CH,CH,),N 10.75 Aromatic Amines Aniline -NH, 4.63 4-Methylaniline CH, -NH2 5.08 4-Chloroanline CI- NH2 4.15 4-Nitroanlline O,N- -NH, 1.0 Aromatic Heterocyclic Amines Pyridine 5.25 Imidazole 6.95
PhC=CH + NH3 = Phenylacetylene Ammonia Table 23.2 Acid Strengths, pK, of the Conjugate Acids of Selected Amines Amine Structure pk, of Conjugate Acld Ammonia NH, 9.26 Primary Amines Methylamine CH,NH, 10.64 Ethylamine CH,CH,NH, 10.81 Cyclohexylamine CH,NH, 10.66 Secondary Amines Dimethylamlne (CH),NH 10.73 Diethylamine (CH,CH,),NH 10.98 Tertiary Amines Trimethylamine (CH,),N 9.81 Triethylamine (CH,CH,),N 10.75 Aromatic Amines Aniline -NH, 4.63 4-Methylaniline CH, -NH2 5.08 4-Chloroanline CI- NH2 4.15 4-Nitroanlline O,N- -NH, 1.0 Aromatic Heterocyclic Amines Pyridine 5.25 Imidazole 6.95
Chapter22: Carbonyl Alpha-substitution Reactions
Section22.1: Keto–enol Tautomerism
Problem 1P
Related questions
Question
Complete the following acid-base reactions and predict the direction of equilibrium (to the right or to the left) for each. Justify your prediction by citing values of pKa for the stronger and weaker acid in each equilibrium. For values of acid ionization constants, consult Table 23.2 (Acid Strengths, pKa, of the Conjugate Acids of Selected
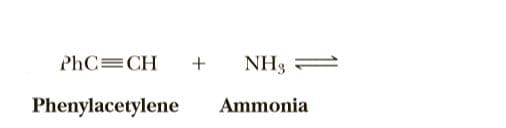
Transcribed Image Text:PhC=CH
+
NH3 =
Phenylacetylene
Ammonia
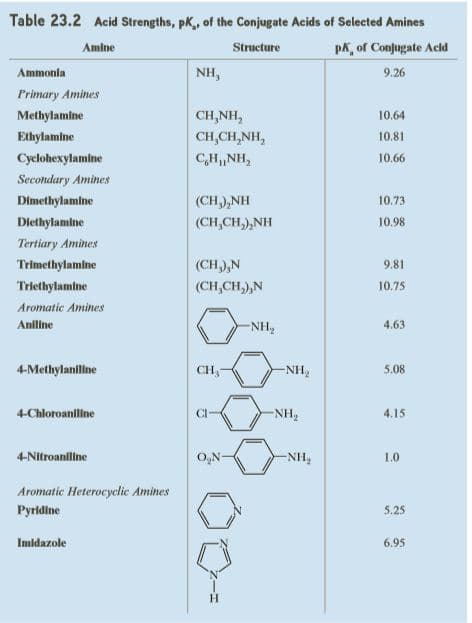
Transcribed Image Text:Table 23.2 Acid Strengths, pK, of the Conjugate Acids of Selected Amines
Amine
Structure
pk, of Conjugate Acld
Ammonia
NH,
9.26
Primary Amines
Methylamine
CH,NH,
10.64
Ethylamine
CH,CH,NH,
10.81
Cyclohexylamine
CH,NH,
10.66
Secondary Amines
Dimethylamlne
(CH),NH
10.73
Diethylamine
(CH,CH,),NH
10.98
Tertiary Amines
Trimethylamine
(CH,),N
9.81
Triethylamine
(CH,CH,),N
10.75
Aromatic Amines
Aniline
-NH,
4.63
4-Methylaniline
CH,
-NH2
5.08
4-Chloroanline
CI-
NH2
4.15
4-Nitroanlline
O,N-
-NH,
1.0
Aromatic Heterocyclic Amines
Pyridine
5.25
Imidazole
6.95
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

