СООН СООН СООН СООН CI СООН СООН NO2 CI `NO2 ČI pk = 3.83 pK, = 3.99 pK. = 2.17 pK, = 3.49 NO2 pк, — 3.44 pK =2.94 СООН СООН СООН NH2 NH2 pK, =4.95 NH2 pK, =4.89 pK=4.73
СООН СООН СООН СООН CI СООН СООН NO2 CI `NO2 ČI pk = 3.83 pK, = 3.99 pK. = 2.17 pK, = 3.49 NO2 pк, — 3.44 pK =2.94 СООН СООН СООН NH2 NH2 pK, =4.95 NH2 pK, =4.89 pK=4.73
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter8: Gases
Section: Chapter Questions
Problem 155CP: Methane (CH4) gas flows into a combustion chamber at a rate of 200. L/min at 1.50 atm and ambient...
Related questions
Question
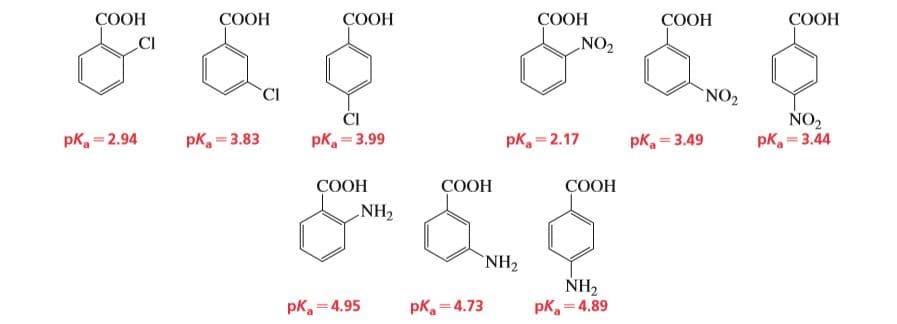
The pKa values of a few ortho-, meta-, and para-substituted benzoic acids are shown below: The relative pKa values depend on the substituent. For chloro-substituted benzoic acids, the ortho isomer is the most acidic and the para isomer is the least acidic; for nitro-substituted benzoic acids, the ortho isomer is the most acidic and the meta isomer is the least acidic; and for amino-substituted benzoic acids, the meta isomer is the most acidic and the ortho isomer is the least acidic. Explain these relative acidities. a. Cl: ortho > meta > para b. NO2: ortho > para > meta c. NH2: meta > para > ortho
Transcribed Image Text:СООН
СООН
СООН
СООН
CI
СООН
СООН
NO2
CI
`NO2
ČI
pk = 3.83
pK, = 3.99
pK. = 2.17
pK, = 3.49
NO2
pк, — 3.44
pK =2.94
СООН
СООН
СООН
NH2
NH2
pK, =4.95
NH2
pK, =4.89
pK=4.73
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

