NH2 Ph. + ОН ÓH 1-Phenyl-2- propanamine (Amphetamine) 2-Hydroxypropanoic acid (Lactic acid) Table 23.2 Acid Strengths, pK, of the Conjugate Acids of Selected Amines Amine Structure pk, of Conjugate Acld Ammonia NH, 9.26 Primary Amines Methylamine CH,NH, 10.64 Ethylamine CH,CH,NH, 10.81 Cyclohexylamine CH,NH, 10.66 Secondary Amines Dimethylamlne (CH),NH 10.73 Diethylamine (CH,CH,),NH 10.98 Tertiary Amines Trimethylamine (CH,),N 9.81 Triethylamine (CH,CH,),N 10.75 Aromatic Amines Aniline -NH, 4.63 4-Methylaniline CH, -NH2 5.08 4-Chloroanline CI- NH2 4.15 4-Nitroanlline O,N- -NH, 1.0 Aromatic Heterocyclic Amines Pyridine 5.25 Imidazole 6.95
NH2 Ph. + ОН ÓH 1-Phenyl-2- propanamine (Amphetamine) 2-Hydroxypropanoic acid (Lactic acid) Table 23.2 Acid Strengths, pK, of the Conjugate Acids of Selected Amines Amine Structure pk, of Conjugate Acld Ammonia NH, 9.26 Primary Amines Methylamine CH,NH, 10.64 Ethylamine CH,CH,NH, 10.81 Cyclohexylamine CH,NH, 10.66 Secondary Amines Dimethylamlne (CH),NH 10.73 Diethylamine (CH,CH,),NH 10.98 Tertiary Amines Trimethylamine (CH,),N 9.81 Triethylamine (CH,CH,),N 10.75 Aromatic Amines Aniline -NH, 4.63 4-Methylaniline CH, -NH2 5.08 4-Chloroanline CI- NH2 4.15 4-Nitroanlline O,N- -NH, 1.0 Aromatic Heterocyclic Amines Pyridine 5.25 Imidazole 6.95
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Andrei Straumanis
ChapterNW2: Nomenclature Worksheet 2: Intro To Naming Functional Groups
Section: Chapter Questions
Problem 17CTQ
Related questions
Question
Complete the following acid-base reactions and predict the direction of equilibrium (to the right or to the left) for each. Justify your prediction by citing values of pKa for the stronger and weaker acid in each equilibrium. For values of acid ionization constants, consult Table 23.2 (Acid Strengths, pKa, of the Conjugate Acids of Selected
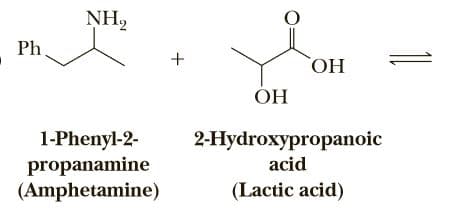
Transcribed Image Text:NH2
Ph.
+
ОН
ÓH
1-Phenyl-2-
propanamine
(Amphetamine)
2-Hydroxypropanoic
acid
(Lactic acid)
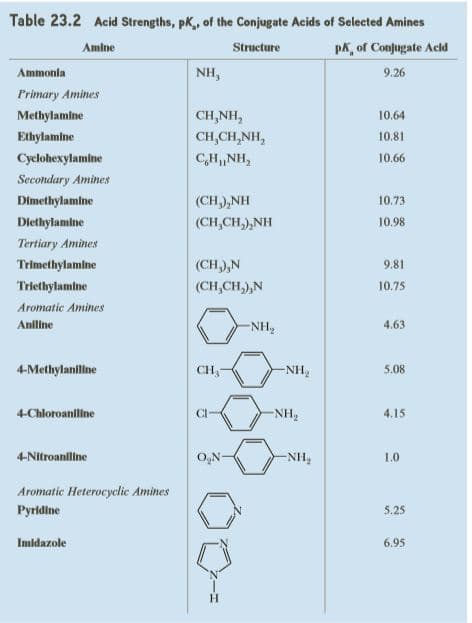
Transcribed Image Text:Table 23.2 Acid Strengths, pK, of the Conjugate Acids of Selected Amines
Amine
Structure
pk, of Conjugate Acld
Ammonia
NH,
9.26
Primary Amines
Methylamine
CH,NH,
10.64
Ethylamine
CH,CH,NH,
10.81
Cyclohexylamine
CH,NH,
10.66
Secondary Amines
Dimethylamlne
(CH),NH
10.73
Diethylamine
(CH,CH,),NH
10.98
Tertiary Amines
Trimethylamine
(CH,),N
9.81
Triethylamine
(CH,CH,),N
10.75
Aromatic Amines
Aniline
-NH,
4.63
4-Methylaniline
CH,
-NH2
5.08
4-Chloroanline
CI-
NH2
4.15
4-Nitroanlline
O,N-
-NH,
1.0
Aromatic Heterocyclic Amines
Pyridine
5.25
Imidazole
6.95
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning