Chapter31: Introduction To Analytical Separations
Section: Chapter Questions
Problem 31.32QAP
Related questions
Question
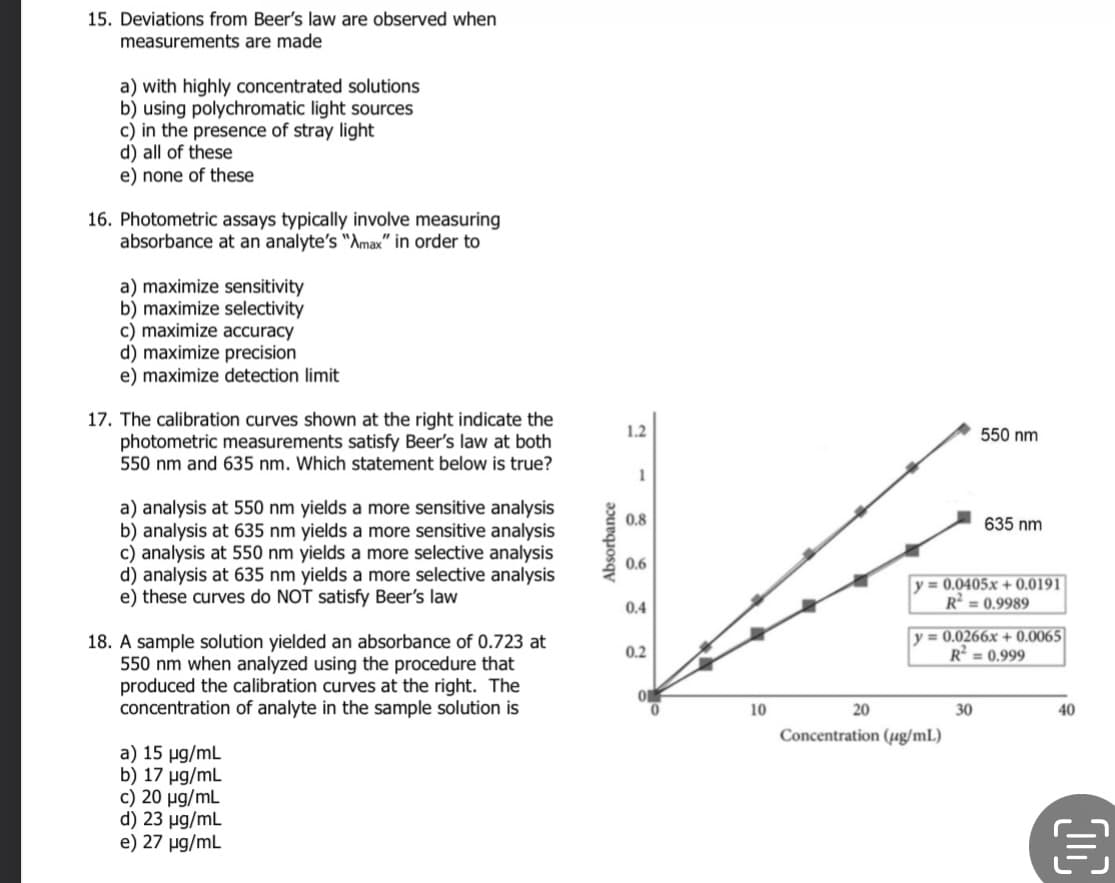
Transcribed Image Text:15. Deviations from Beer's law are observed when
measurements are made
a) with highly concentrated solutions
b) using polychromatic light sources
c) in the presence of stray light
d) all of these
e) none of these
16. Photometric assays typically involve measuring
absorbance at an analyte's "Amax" in order to
a) maximize sensitivity
b) maximize selectivity
c) maximize accuracy
d) maximize precision
e) maximize detection limit
17. The calibration curves shown at the right indicate the
photometric measurements satisfy Beer's law at both
550 nm and 635 nm. Which statement below is true?
a) analysis at 550 nm yields a more sensitive analysis
b) analysis at 635 nm yields a more sensitive analysis
c) analysis at 550 nm yields a more selective analysis
d) analysis at 635 nm yields a more selective analysis
e) these curves do NOT satisfy Beer's law
18. A sample solution yielded an absorbance of 0.723 at
550 nm when analyzed using the procedure that
produced the calibration curves at the right. The
concentration of analyte in the sample solution is
a) 15 µg/mL
b) 17 µg/mL
c) 20 µg/mL
d) 23 µg/mL
e) 27 µg/mL
Absorbance
1.2
1
0.8
0.6
0.4
0.2
0
10
20
550 nm
y=0.0405x+0.0191
R=0.9989
Concentration (µg/mL)
635 nm
y= 0.0266x+0.0065
R² = 0.999
30
40
D

Transcribed Image Text:10. Photoluminescence
is a term describing the emission
of light by atoms or molecules that have been excited
by
energy.
a) chemical
b) electrical
c) electromagnetic
d) thermal
e) any of these
11. Which of the equalities below is the definition of the
property "absorbance"?
a) A = abc
b) A = Ebc
c) A = -log T
d) all of these
e) none of these
12. Under certain conditions the transmittance of a sample
is measured to be 79%. What is the corresponding
absorbance of this sample?
a) 0.00
b) 0.10
c) 0.21
d) 0.79
e) 1.00
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 5 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you



