PHOTOSYNTHETIC PIGMENTS 1.6 0.7 1.4 0.6 1.2E Soret 0.5 HA 0.4 0.8 COOCH 0.3 0.6 phytyl bacteriochlorophyll a 0.2- 0.4 Qx 0.1 0.2 0. 300 400 500 600 700 800 700 750 800 850 900 2 (nm) 2 (nm) Figure 4.7 Absorption (left) and fluorescence (right) spectra of bacteriochlorophyll a in diethyl ether. Absorbance C Fluorescence (Arbitrary units)
PHOTOSYNTHETIC PIGMENTS 1.6 0.7 1.4 0.6 1.2E Soret 0.5 HA 0.4 0.8 COOCH 0.3 0.6 phytyl bacteriochlorophyll a 0.2- 0.4 Qx 0.1 0.2 0. 300 400 500 600 700 800 700 750 800 850 900 2 (nm) 2 (nm) Figure 4.7 Absorption (left) and fluorescence (right) spectra of bacteriochlorophyll a in diethyl ether. Absorbance C Fluorescence (Arbitrary units)
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter20: Molecular Spectroscopy And Photochemistry
Section: Chapter Questions
Problem 65AP
Related questions
Question
100%
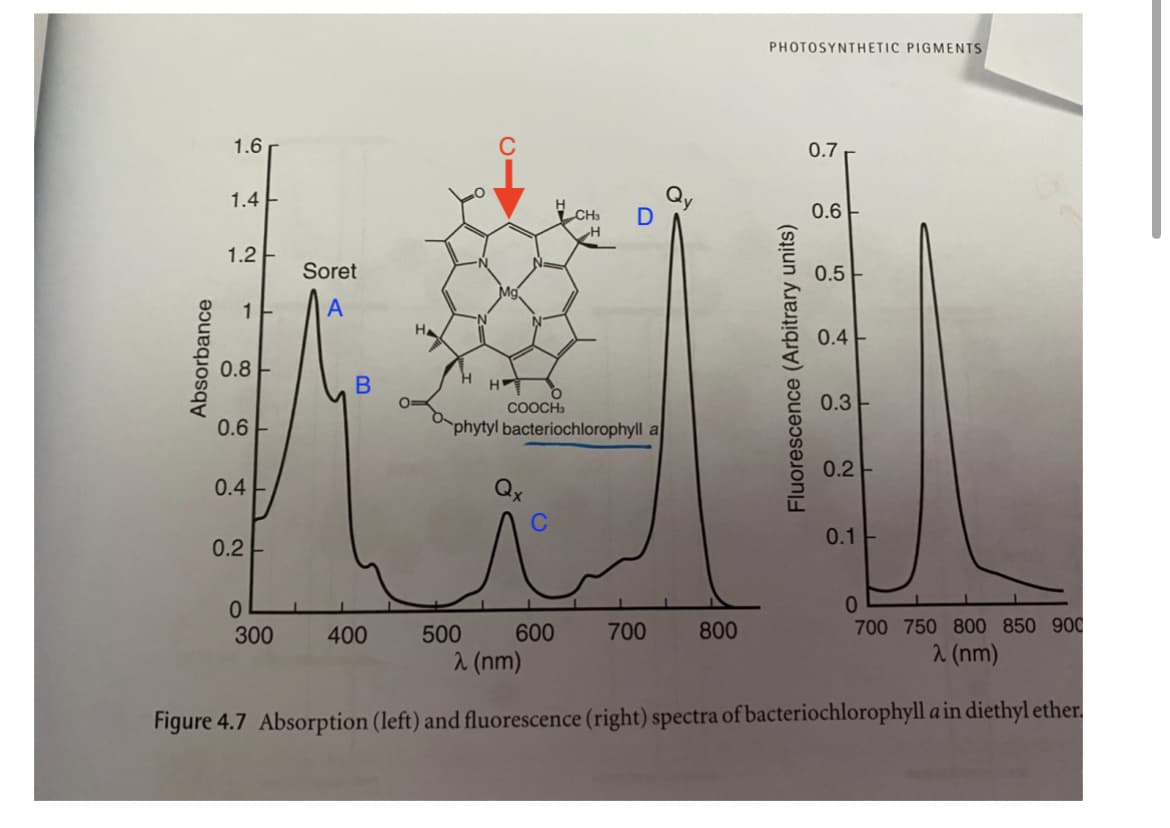
Transcribed Image Text:PHOTOSYNTHETIC PIGMENTS
1.6
0.7
Qy
D
1.4
0.6
1.2 E
Soret
0.5-
1
A
N-
0.4
0.8
H.
H
0.3
COOCH3
0.6
phytyl bacteriochlorophyll a
0.2-
0.4
Qx
0.1
0.2
300
400
500
600
700
800
700 750 800 850 900
2 (nm)
2 (nm)
Figure 4.7 Absorption (left) and fluorescence (right) spectra of bacteriochlorophyll a in diethyl ether.
Absorbance
B
C
Fluorescence (Arbitrary units)
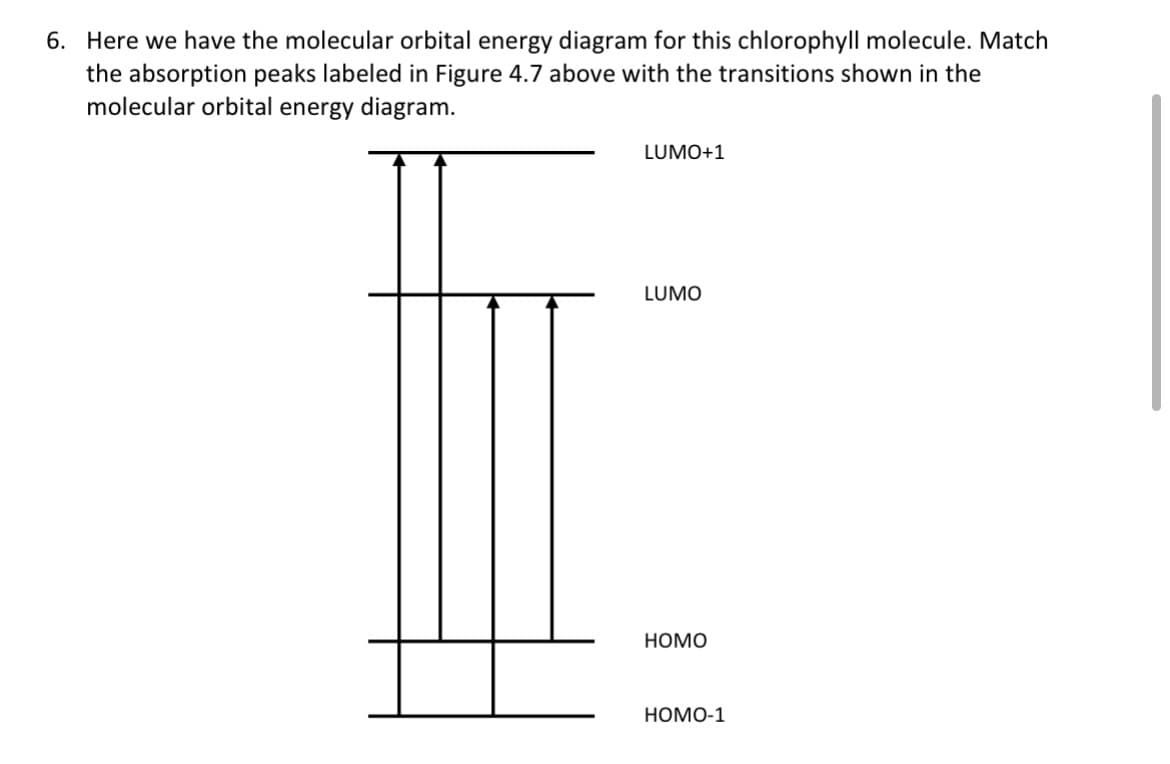
Transcribed Image Text:6. Here we have the molecular orbital energy diagram for this chlorophyll molecule. Match
the absorption peaks labeled in Figure 4.7 above with the transitions shown in the
molecular orbital energy diagram.
LUMO+1
LUMO
HOMO
НОМО-1
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning