Chapter10: Effect Of Electrolytes On Chemical Equilibria
Section: Chapter Questions
Problem 10.8QAP
Related questions
Question
Please answer all parts
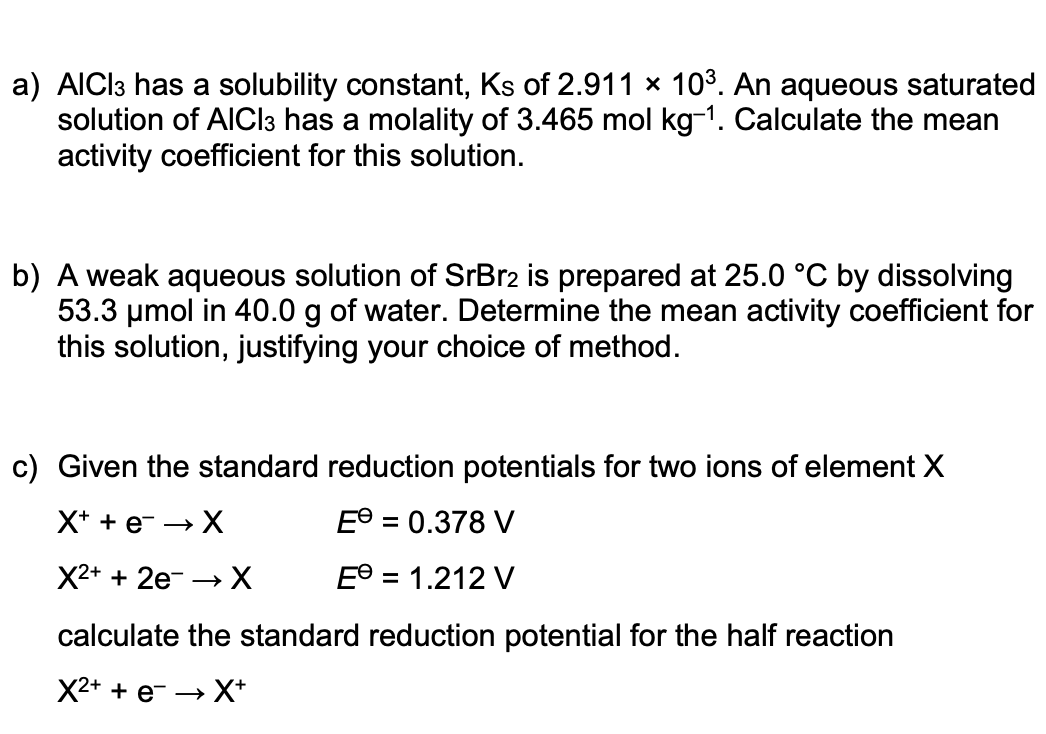
Transcribed Image Text:a) AICI3 has a solubility constant, Ks of 2.911 × 103. An aqueous saturated
solution of AICI3 has a molality of 3.465 mol kg-1. Calculate the mean
activity coefficient for this solution.
b) A weak aqueous solution of SrBr2 is prepared at 25.0 °C by dissolving
53.3 umol in 40.0 g of water. Determine the mean activity coefficient for
this solution, justifying your choice of method.
c) Given the standard reduction potentials for two ions of element X
X+ + e- → X
E® = 0.378 V
X2+ + 2e- → X
E° = 1.212 V
calculate the standard reduction potential for the half reaction
X2+ + e- → X*
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

