Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter14: Acid- Base Equilibria
Section: Chapter Questions
Problem 118CP: Malonic acid (HO2CCH2CO2H) is a diprotic acid. In the titration of malonic acid w ith NaOH,...
Related questions
Question
Please help me fill out the rest of the chart. Show work.
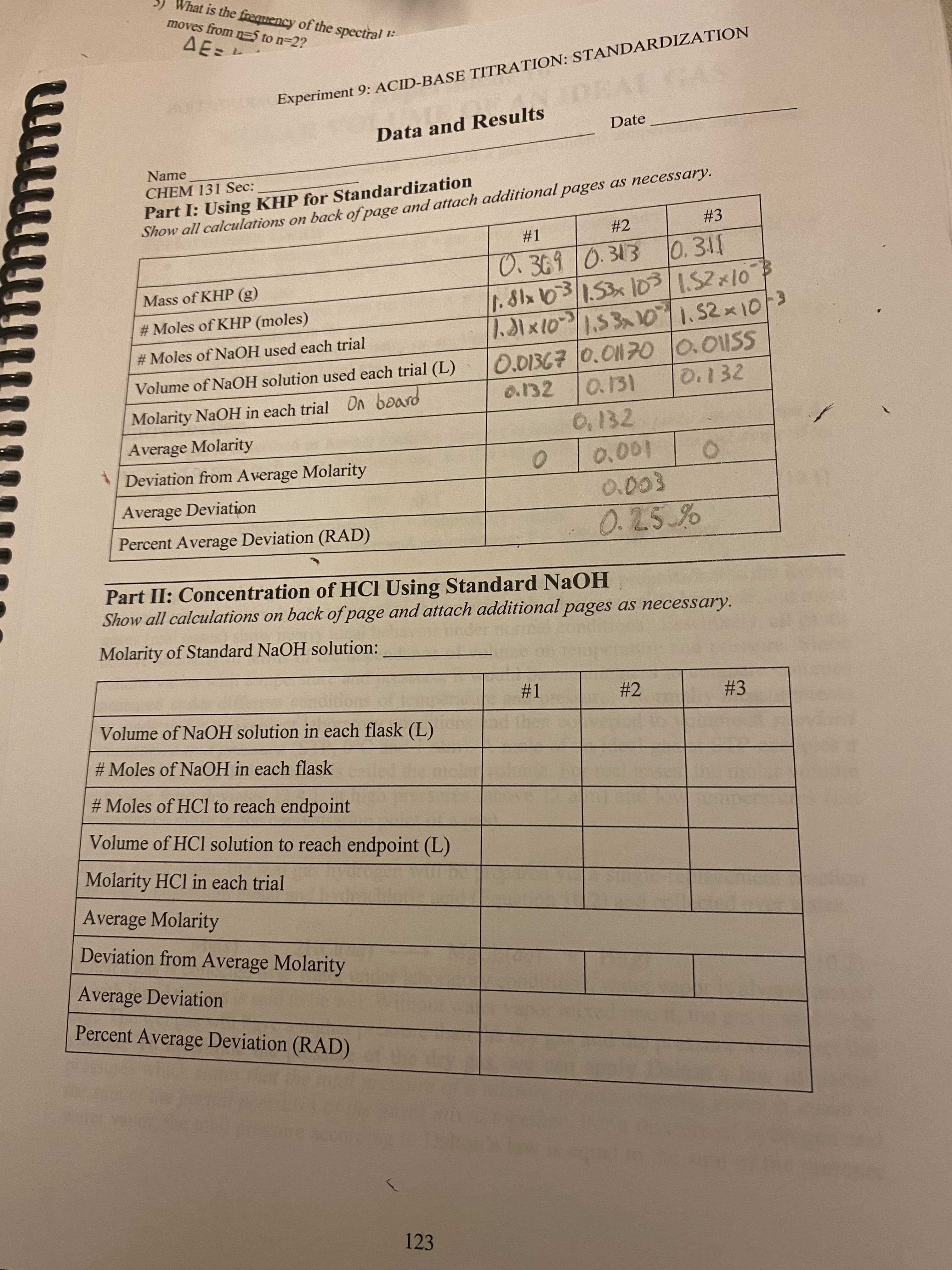
Transcribed Image Text:1:
Experiment 9: ACID-BASE TITRATION: STANDARDIZATION
What is the frequency of the spectral
moves from n=5 to n=2?
ΔΕΞ
Data and Results
Date
Name
CHEM 131 Sec:
Part I: Using KHP for Standardization
Show all calculations on back of page and attach additional pages as necessary.
#2
#3
#1
0.369 0.313
0.311
Mass of KHP (g)
1.81x 103 / 1.53 x 10³
1.52 x 10
#Moles of KHP (moles)
1.31 × 10³ | 1.53x10 | 1.52 × 10/³
#Moles of NaOH used each trial
0.01367 0.01170
0.01SS
Volume of NaOH solution used each trial (L)
0.132
0.132 0.131
Molarity NaOH in each trial On board
0.132
Average Molarity
Deviation from Average Molarity
0
0.001
Average Deviation
0.003
0.25%
Percent Average Deviation (RAD)
Part II: Concentration of HCI Using Standard NaOH
Show all calculations on back of page and attach additional pages as necessary.
Molarity of Standard NaOH solution:
#1
#2
#3
Volume of NaOH solution in each flask (L)
#Moles of NaOH in each flask
#Moles of HCl to reach endpoint
Volume of HCl solution to reach endpoint (L)
Molarity HCl in each trial
Average Molarity
Deviation from Average Molarity
Average Deviation
Percent Average Deviation (RAD)
(
123
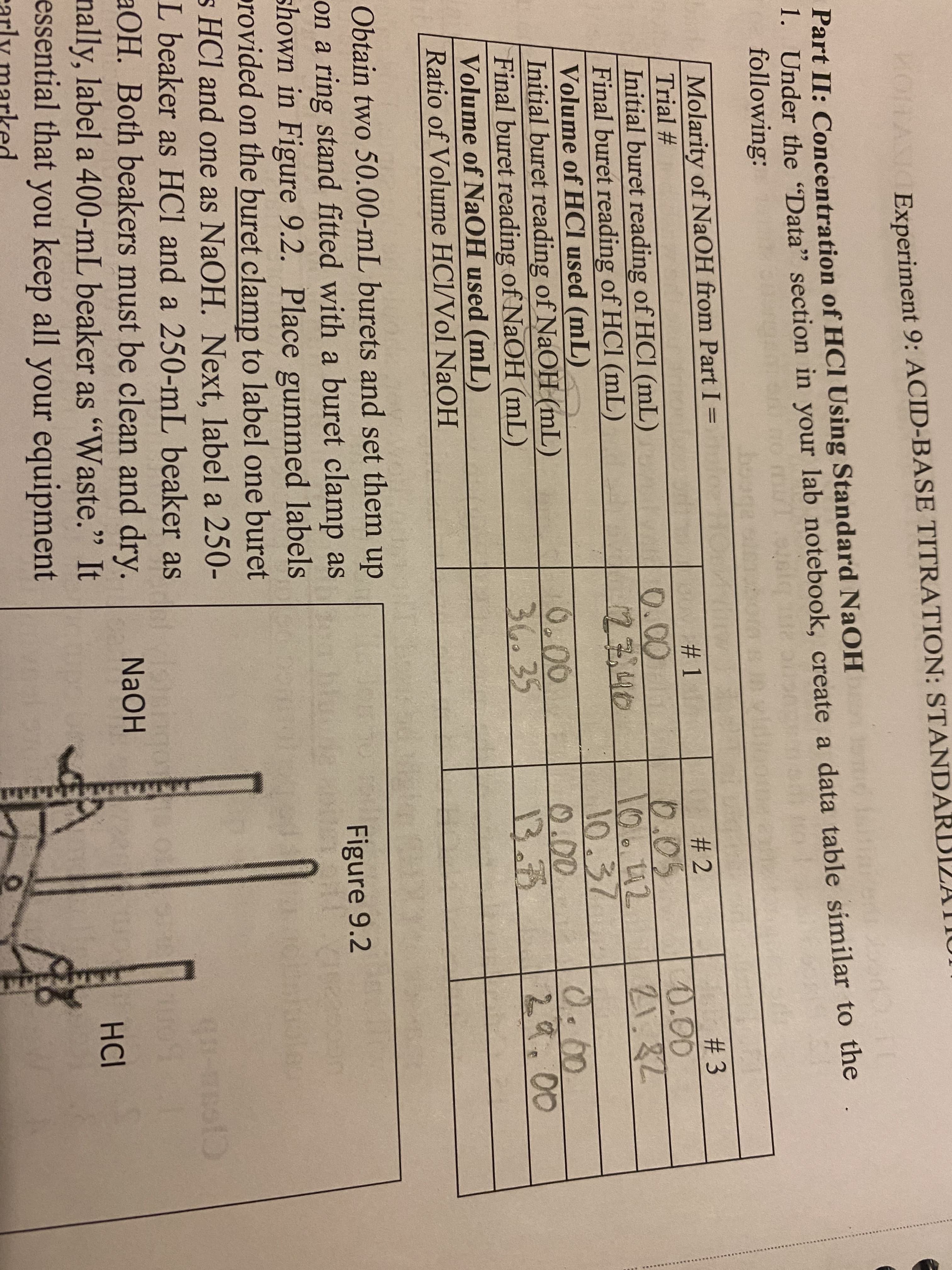
Transcribed Image Text:MOITASIOExperiment 9: ACID-BASE TITRATION: STANDA
Part II: Concentration of HCI Using Standard NaOH
1. Under the "Data" section in your lab notebook, create a data table similar to the
following:
zeigte
booga sluzo
othe
HOM
Molarity of NaOH from Part I =
Trial #
#2
#3
0.05
0.00
Initial buret reading of HCl (mL)
Final buret reading of HCl (mL)
Volume of HCI used (mL)
106.42
21.82
10.37
0.00
0.00
Initial buret reading of NaOH (mL)
Final buret reading of NaOH (mL)
Volume of NaOH used (mL)
Ratio of Volume HCl/Vol NaOH
13.72
29.00
Figure 9.2
Til
Obtain two 50.00-mL burets and set them up
on a ring stand fitted with a buret clamp as
shown in Figure 9.2. Place gummed labels
provided on the buret clamp to label one buret
s HCl and one as NaOH. Next, label a 250-
L beaker as HCl and a 250-mL beaker as
aOH. Both beakers must be clean and dry.
nally, label a 400-mL beaker as "Waste." It
essential that you keep all your equipment
carly marked
#1
0.00
12340
0.00
36.35
singos
NaOH
WW
O
-Esto
HCI
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning


Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning