Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter11: Liquids And Solids
Section: Chapter Questions
Problem 11.74QE
Related questions
Question
Please show step-by-step solution.
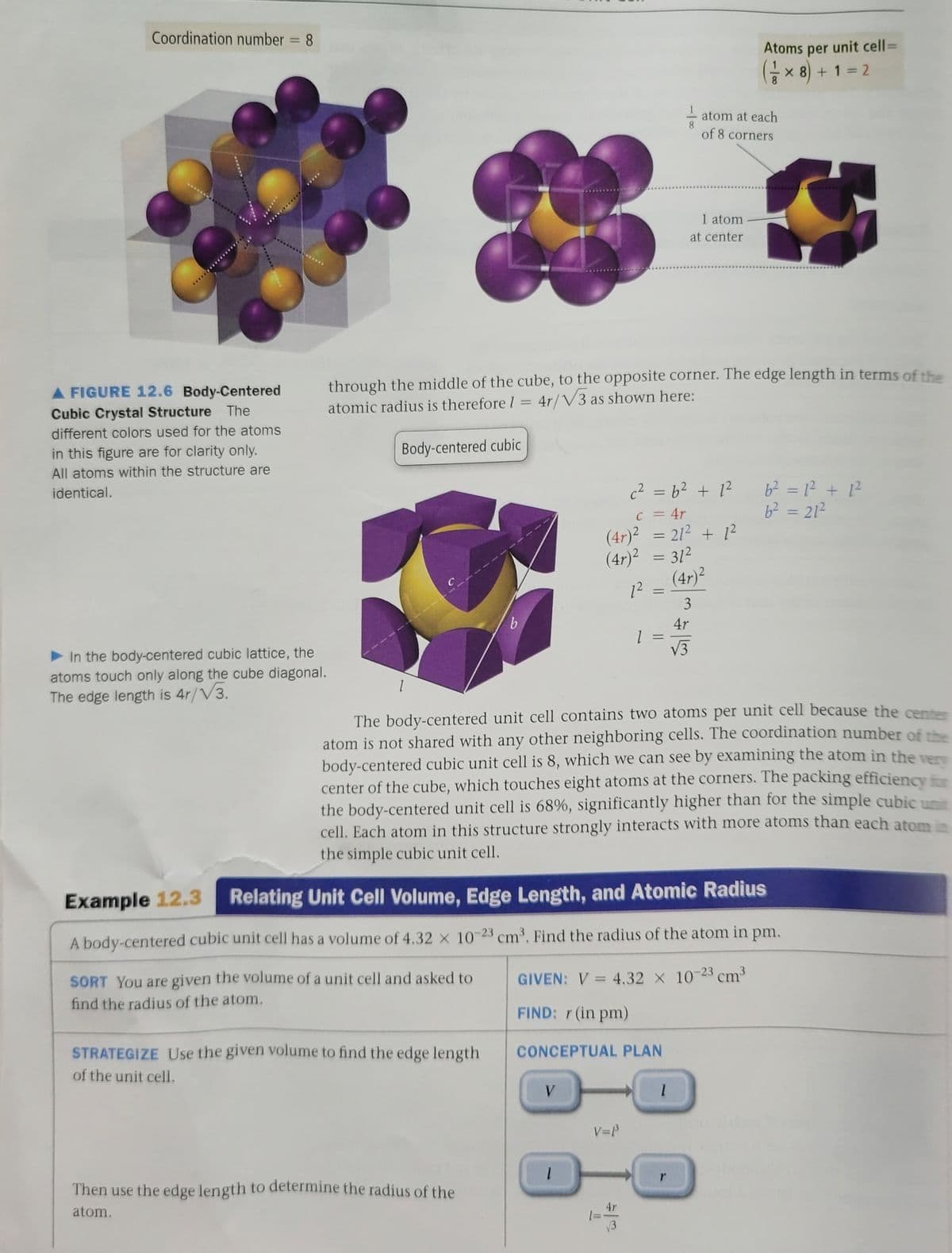
Transcribed Image Text:Coordination number = 8
%3D
Atoms per unit cell=
Gx 8) + 1 = 2
atom at each
8.
of 8 corners
...............
1 atom
at center
through the middle of the cube, to the opposite corner. The edge length in terms of the
atomic radius is therefore 1 = 4r/V3 as shown here:
A FIGURE 12.6 Body-Centered
Cubic Crystal Structure The
%3D
different colors used for the atoms
in this figure are for clarity only.
Body-centered cubic
All atoms within the structure are
identical.
c2 = b2 + 1?
6? = 1² + 1?
b2 = 212
%3D
c = 4r
(4r)² = 21² + 1?
(4r)² = 31²
(4r)2
%3D
%3D
12
4r
In the body-centered cubic lattice, the
atoms touch only along the cube diagonal.
The edge length is 4r/V3.
V3
The body-centered unit cell contains two atoms per unit cell because the cente
atom is not shared with any other neighboring cells. The coordination number of th
body-centered cubic unit cell is 8, which we can see by examining the atom in the e
center of the cube, which touches eight atoms at the corners. The packing efficiency
the body-centered unit cell is 68%, significantly higher than for the simple cubic u
cell. Each atom in this structure strongly interacts with more atoms than each atom
the simple cubic unit cell.
Example 12.3
Relating Unit Cell Volume, Edge Length, and Atomic Radius
A body-centered cubic unit cell has a volume of 4.32 x 10-23 cm³. Find the radius of the atom in pm.
SORT You are given the volume of a unit cell and asked to
GIVEN: V = 4.32 X 10-23 cm³
%3D
find the radius of the atom.
FIND: r (in pm)
STRATEGIZE Use the given volume to find the edge length
CONCEPTUAL PLAN
of the unit cell.
V
V=
Then use the edge length to determine the radius of the
atom.
4r
V3
****

Transcribed Image Text:5) An atom has radius of 165 pm and crystallizes in the body-centered cubic unit cell. What is
the volume of the unit cell in cm³? You will need to use examples from your textbook to
help you. Pg 540-541
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning