Polyacrylamide gels (PAGS) are made by mixing acrylamide with bisacrylamide as a crosslinker. The amount of acrylamide and bisacrylamide in a gel is expressed as a percentage in 100 mL of buffer. PAGS can have a uniform, single, and continuous percentage throughout the gel (single-percentage gels), or they can be cast with an increasing gradient of acrylamide and bisacrylamide across the gel (gradient gels). Typical gel compositions are between 7.5 and 20% for single-percentage gels, and typical gradients are 4-15% and 10-20%. Observe the results below and compare them within each class of gels across different percentages, and between the two different classes. Provide your comments on which one is your favorite and why? Please be concise and brief. Single percentage Gradient Tris-HCI 12.5% 15% 18% 4-15% 4-20% 8-16% 10-20% 10.5-14% 5% 7.5% 10% 250 150 250 150 250 250 100 150 100 250 100 100 250 150 75 250 100 75 150 75 75 75 50 100 150 50 37 150 100 50 250 37 25 50 75 50 20 100 75 100 37 37 75 50 37 25 15 25 50 37 150 75 20 50 20 25 25 20 10 37 20 25 37 25 15 15 15 15 20 20 50 100 10 10 10 15 10 15 10 10 37 25 75 Edit View Insert Format Tools Table || | 1 38 29
Polyacrylamide gels (PAGS) are made by mixing acrylamide with bisacrylamide as a crosslinker. The amount of acrylamide and bisacrylamide in a gel is expressed as a percentage in 100 mL of buffer. PAGS can have a uniform, single, and continuous percentage throughout the gel (single-percentage gels), or they can be cast with an increasing gradient of acrylamide and bisacrylamide across the gel (gradient gels). Typical gel compositions are between 7.5 and 20% for single-percentage gels, and typical gradients are 4-15% and 10-20%. Observe the results below and compare them within each class of gels across different percentages, and between the two different classes. Provide your comments on which one is your favorite and why? Please be concise and brief. Single percentage Gradient Tris-HCI 12.5% 15% 18% 4-15% 4-20% 8-16% 10-20% 10.5-14% 5% 7.5% 10% 250 150 250 150 250 250 100 150 100 250 100 100 250 150 75 250 100 75 150 75 75 75 50 100 150 50 37 150 100 50 250 37 25 50 75 50 20 100 75 100 37 37 75 50 37 25 15 25 50 37 150 75 20 50 20 25 25 20 10 37 20 25 37 25 15 15 15 15 20 20 50 100 10 10 10 15 10 15 10 10 37 25 75 Edit View Insert Format Tools Table || | 1 38 29
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter20: Molecular Spectroscopy And Photochemistry
Section: Chapter Questions
Problem 67CP
Related questions
Question
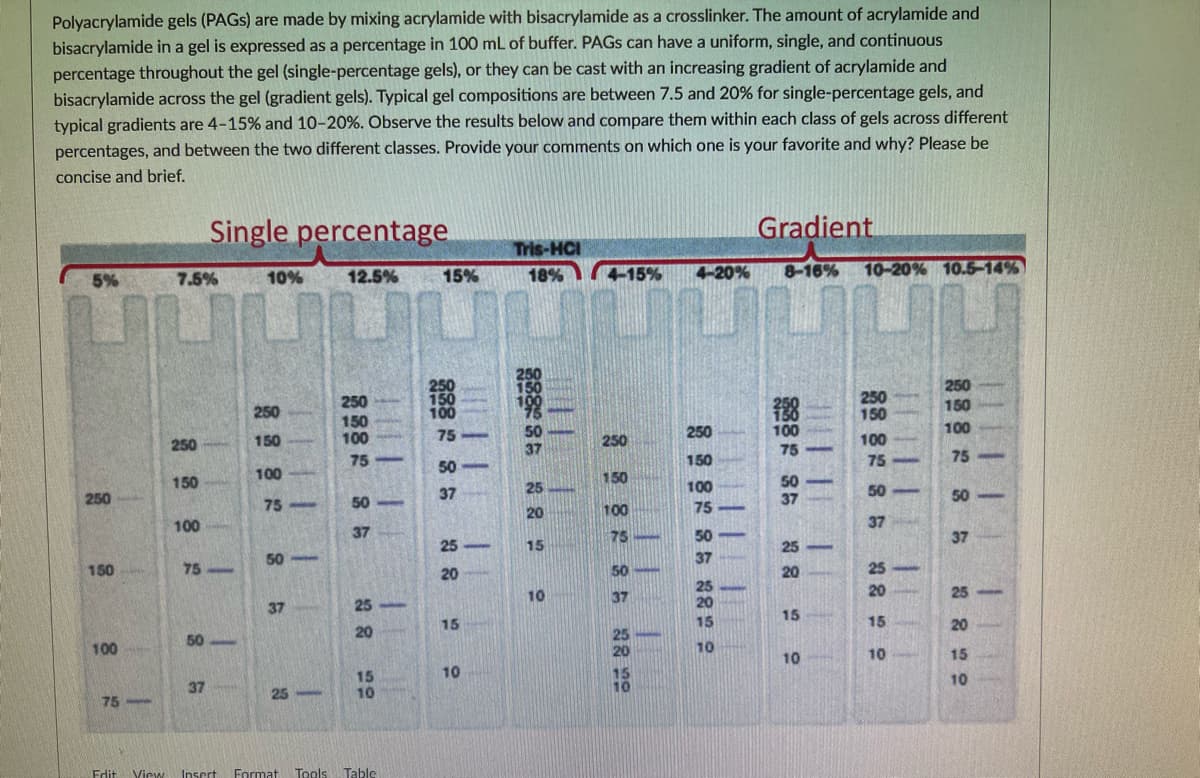
Transcribed Image Text:Polyacrylamide gels (PAGS) are made by mixing acrylamide with bisacrylamide as a crosslinker. The amount of acrylamide and
bisacrylamide in a gel is expressed as a percentage in 100 mL of buffer. PAGS can have a uniform, single, and continuous
percentage throughout the gel (single-percentage gels), or they can be cast with an increasing gradient of acrylamide and
bisacrylamide across the gel (gradient gels). Typical gel compositions are between 7.5 and 20% for single-percentage gels, and
typical gradients are 4-15% and 10-20%. Observe the results below and compare them within each class of gels across different
percentages, and between the two different classes. Provide your comments on which one is your favorite and why? Please be
concise and brief.
Single percentage
Gradient
Tris-HCI
12.5%
15%
18%
4-15%
4-20%
8-16%
10-20% 10.5-14%
5%
7.5%
10%
250
250
250
150
150
250
100
150
100
250
100
100
250
150
75
250
100
75
75
150
75
75
50
100
150
50
37
150
25
100
50
250
37
50
75
50
20
100
75
100
37
37
75
50
37
25 -
15
25
50
37
150
75
20
50
20
25
25
20
20
25
10
37
37
25
15
15
15
15
20
20
50
100
10
10
10
15
10
15
10
10
37
25
75
Edit View Insert Format
Tools
Table
|| | |
38 29
Expert Solution
Step 1
Single-percentage gels are used to separate bands that are close in molecular weight. Optimum separation occurs in the lower half of the gel, so the protein to be used migrates to the lower half of the gel and separated from the other protein and biomolecules.
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning