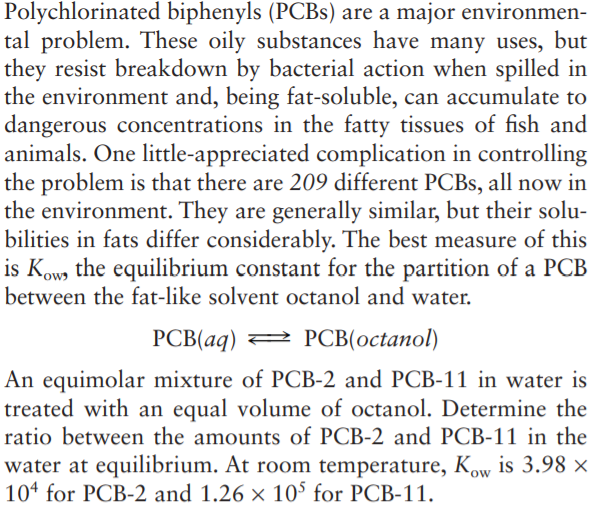
Polychlorinated biphenyls (PCBS) are a major environmen- tal problem. These oily substances have many uses, but they resist breakdown by bacterial action when spilled in the environment and, being fat-soluble, can accumulate to dangerous concentrations in the fatty tissues of fish and animals. One little-appreciated complication in controlling the problem is that there are 209 different PCBS, all now in the environment. They are generally similar, but their solu- bilities in fats differ considerably. The best measure of this is Kow, the equilibrium constant for the partition of a PCB between the fat-like solvent octanol and water. PCB(aq) = 2 PCB(octanol) An equimolar mixture of PCB-2 and PCB-11 in water is treated with an equal volume of octanol. Determine the ratio between the amounts of PCB-2 and PCB-11 in the water at equilibrium. At room temperature, Kow is 3.98 × 104 for PCB-2 and 1.26 × 10° for PCB-11.
Polychlorinated biphenyls (PCBS) are a major environmen- tal problem. These oily substances have many uses, but they resist breakdown by bacterial action when spilled in the environment and, being fat-soluble, can accumulate to dangerous concentrations in the fatty tissues of fish and animals. One little-appreciated complication in controlling the problem is that there are 209 different PCBS, all now in the environment. They are generally similar, but their solu- bilities in fats differ considerably. The best measure of this is Kow, the equilibrium constant for the partition of a PCB between the fat-like solvent octanol and water. PCB(aq) = 2 PCB(octanol) An equimolar mixture of PCB-2 and PCB-11 in water is treated with an equal volume of octanol. Determine the ratio between the amounts of PCB-2 and PCB-11 in the water at equilibrium. At room temperature, Kow is 3.98 × 104 for PCB-2 and 1.26 × 10° for PCB-11.
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter14: Chemical Equilibrium
Section: Chapter Questions
Problem 93AP
Related questions
Question
Transcribed Image Text:Polychlorinated biphenyls (PCBS) are a major environmen-
tal problem. These oily substances have many uses, but
they resist breakdown by bacterial action when spilled in
the environment and, being fat-soluble, can accumulate to
dangerous concentrations in the fatty tissues of fish and
animals. One little-appreciated complication in controlling
the problem is that there are 209 different PCBS, all now in
the environment. They are generally similar, but their solu-
bilities in fats differ considerably. The best measure of this
is Kow, the equilibrium constant for the partition of a PCB
between the fat-like solvent octanol and water.
PCB(aq) =
2 PCB(octanol)
An equimolar mixture of PCB-2 and PCB-11 in water is
treated with an equal volume of octanol. Determine the
ratio between the amounts of PCB-2 and PCB-11 in the
water at equilibrium. At room temperature, Kow is 3.98 ×
104 for PCB-2 and 1.26 × 10° for PCB-11.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 6 steps with 8 images

Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning