Potassium dichromate is used to titrate a sample containing an unknown percentage of iron (AW. 55.845 g/mol ). The sample is dissolved in H3PO4/H2SO4 mixture reduce all of the iron to Fe<* ions. The solution is then titrated with 0.01625 M K2Cr207, producing Fe* and Cr* ions in acidic solution. The titration requires 32.26 mL of K2Cr207 for 1.2765 g of the sample.
Potassium dichromate is used to titrate a sample containing an unknown percentage of iron (AW. 55.845 g/mol ). The sample is dissolved in H3PO4/H2SO4 mixture reduce all of the iron to Fe<* ions. The solution is then titrated with 0.01625 M K2Cr207, producing Fe* and Cr* ions in acidic solution. The titration requires 32.26 mL of K2Cr207 for 1.2765 g of the sample.
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter22: The Chemistry Of The Transistion Elements
Section22.7: Colors Of Coordination Compounds
Problem 3.3ACP
Related questions
Question
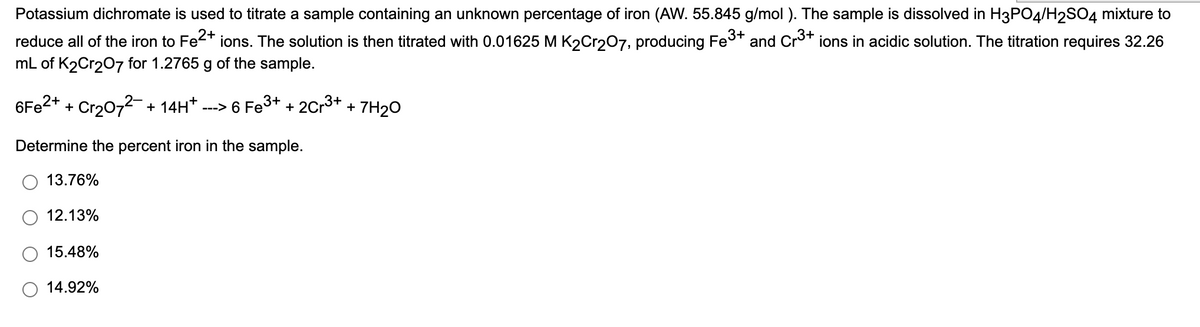
Transcribed Image Text:Potassium dichromate is used to titrate a sample containing an unknown percentage of iron (AW. 55.845 g/mol ). The sample is dissolved in H3PO4/H2SO4 mixture to
reduce all of the iron to Fe2* ions. The solution is then titrated with 0.01625 M K2Cr207, producing Fes+
mL of K2Cr207 for 1.2765 g of the sample.
and Cro+ ions in acidic solution. The titration requires 32.26
6F22+ + Cr2072- + 14H* .
---> 6 Fe3+ + 2Cr3+
7H2O
+
Determine the percent iron in the sample.
13.76%
12.13%
15.48%
14.92%
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax