Potassium hydrogen phthalate (KHP) concentrations can be determined through titrating samples of KHP (a monoprotic acid) with bases such as NaOH in the presence of an indicator such as phenolphthalein. The indicator is colorless in an acidic solution and turns pink in an alkaline solution. Thus, we can establish an equilibrium for the phenolphthalein with the following reaction. HIn + H,0 = In + H,0* If the Hin species is "acid color" or colorless for the phenolphthalein, and the In species is "base color" or pink for this particular indicator, what color will appear in a flask in which a 0.2993 gram sample of KHP is completely neutralized with an excess of NaOH?
Potassium hydrogen phthalate (KHP) concentrations can be determined through titrating samples of KHP (a monoprotic acid) with bases such as NaOH in the presence of an indicator such as phenolphthalein. The indicator is colorless in an acidic solution and turns pink in an alkaline solution. Thus, we can establish an equilibrium for the phenolphthalein with the following reaction. HIn + H,0 = In + H,0* If the Hin species is "acid color" or colorless for the phenolphthalein, and the In species is "base color" or pink for this particular indicator, what color will appear in a flask in which a 0.2993 gram sample of KHP is completely neutralized with an excess of NaOH?
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter15: Acid–base Equilibria
Section: Chapter Questions
Problem 57P
Related questions
Question
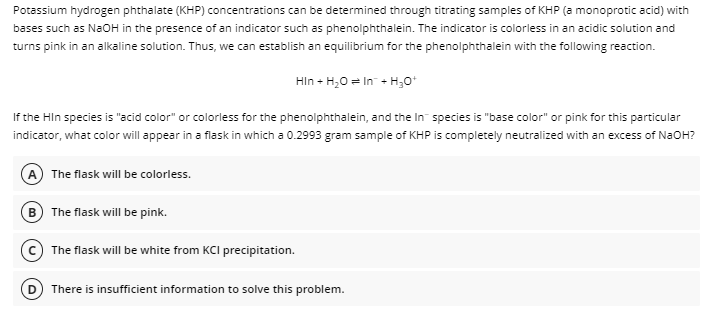
Transcribed Image Text:Potassium hydrogen phthalate (KHP) concentrations can be determined through titrating samples of KHP (a monoprotic acid) with
bases such as NaOH in the presence of an indicator such as phenolphthalein. The indicator is colorless in an acidic solution and
turns pink in an alkaline solution. Thus, we can establish an equilibrium for the phenolphthalein with the following reaction.
HIn + H,0 = In + H;0*
If the Hin species is "acid color" or colorless for the phenolphthalein, and the In species is "base color" or pink for this particular
indicator, what color will appear in a flask in which a 0.2993 gram sample of KHP is completely neutralized with an excess of NaOH?
A The flask will be colorless.
B The flask will be pink.
The flask will be white from KCI precipitation.
There is insufficient information to solve this problem.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning