Potassium nitrate has a lattice energy of -163.8 kcal/mol and a heat of hydration of -155.5 kcal/mol. Part A You may want to reference (Pages 544 - 548) Section 13.3 while completing this problem. How much potassium nitrate has to dissolve in water to absorb 101 kJ of heat? Express your answer using two significant figures. 0.32 kg
Potassium nitrate has a lattice energy of -163.8 kcal/mol and a heat of hydration of -155.5 kcal/mol. Part A You may want to reference (Pages 544 - 548) Section 13.3 while completing this problem. How much potassium nitrate has to dissolve in water to absorb 101 kJ of heat? Express your answer using two significant figures. 0.32 kg
Chapter7: Solutions And Colloids
Section: Chapter Questions
Problem 7.9E
Related questions
Question
100%
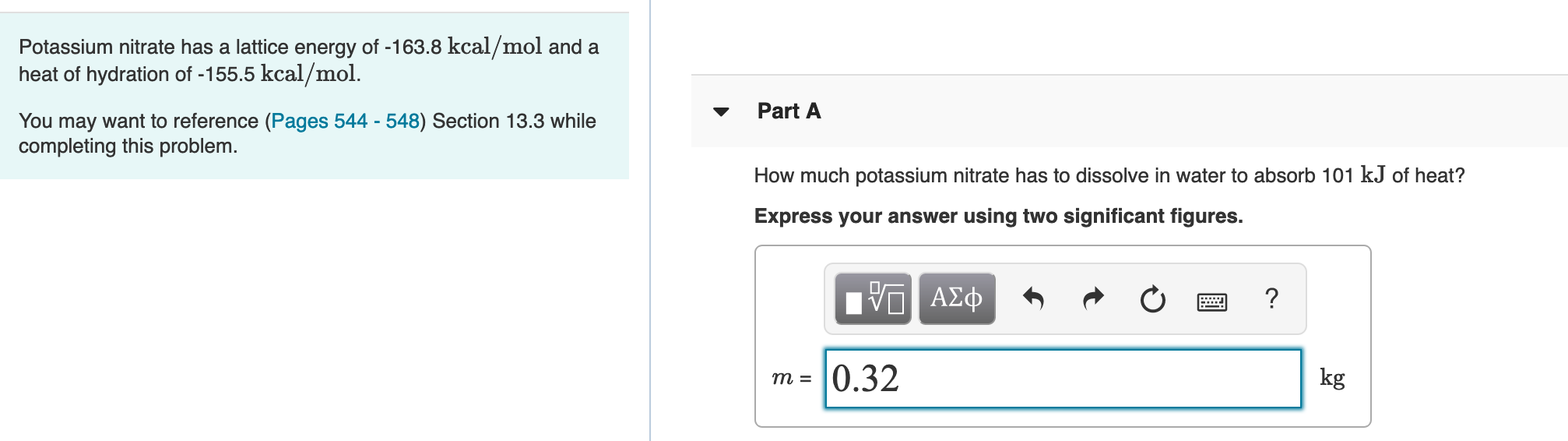
Transcribed Image Text:Potassium nitrate has a lattice energy of -163.8 kcal/mol and a
heat of hydration of -155.5 kcal/mol.
Part A
You may want to reference (Pages 544 - 548) Section 13.3 while
completing this problem.
How much potassium nitrate has to dissolve in water to absorb 101 kJ of heat?
Express your answer using two significant figures.
0.32
kg
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning