potential eneryy progress of reaction /time Identify the graph above as an endothermic or exothermic reaction. Explain how you include details such as the delta H (change in heat), if heat is a reactant or product, ar cause the container to be hot or cold to the touch. here to search
potential eneryy progress of reaction /time Identify the graph above as an endothermic or exothermic reaction. Explain how you include details such as the delta H (change in heat), if heat is a reactant or product, ar cause the container to be hot or cold to the touch. here to search
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter11: Chemical Kinetics
Section: Chapter Questions
Problem 11.99PAE: Substances that poison a catalyst pose a major concern for many engineering designs, including those...
Related questions
Question
Transcribed Image Text:A Translate
R The Latest Girls & G.
O Unit
t Watch Free Movies.
9 Aceable Driving | D.
Tarot Card Meaning..
3
4
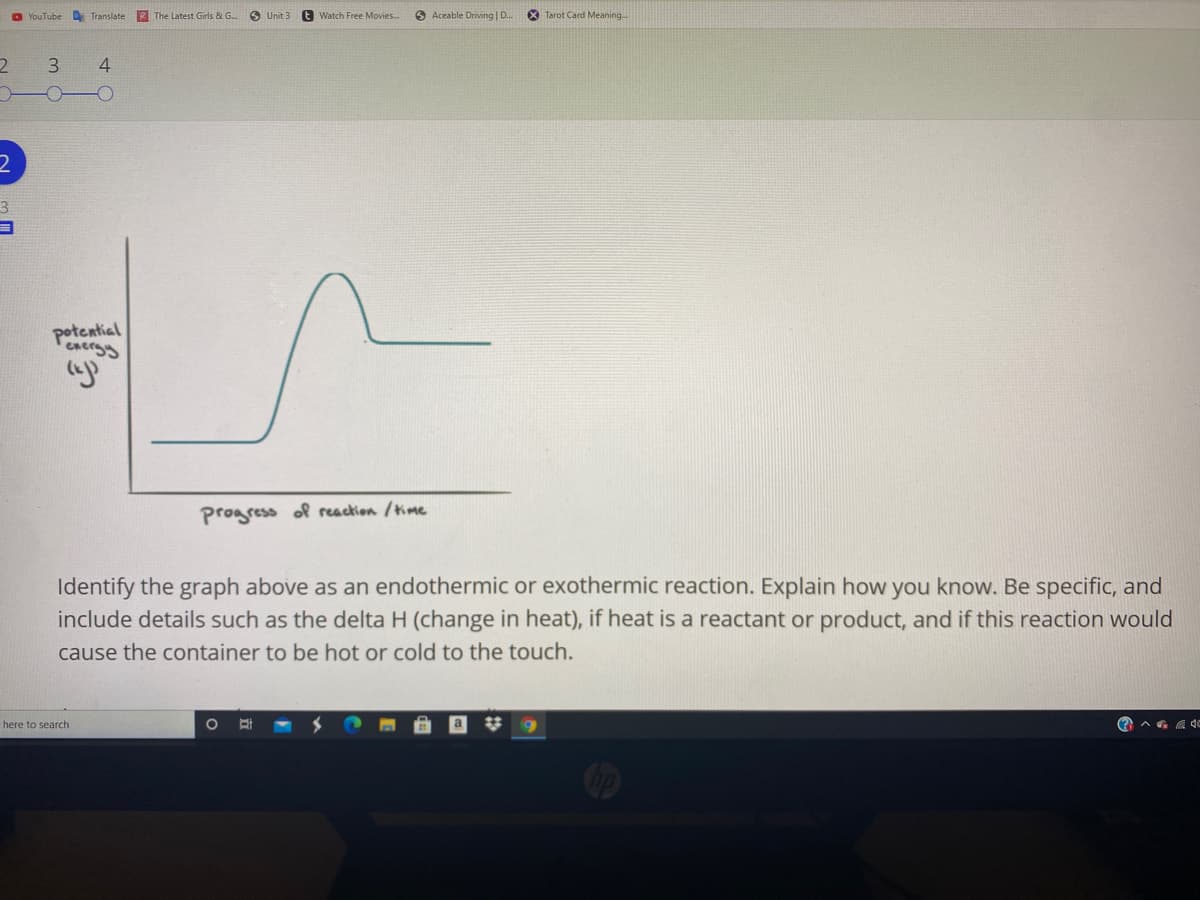
potential
progress of reaction /time
Identify the graph above as an endothermic or exothermic reaction. Explain how you know. Be specific, and
include details such as the delta H (change in heat), if heat is a reactant or product, and if this reaction would
cause the container to be hot or cold to the touch.
here to search
(?
ap
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning