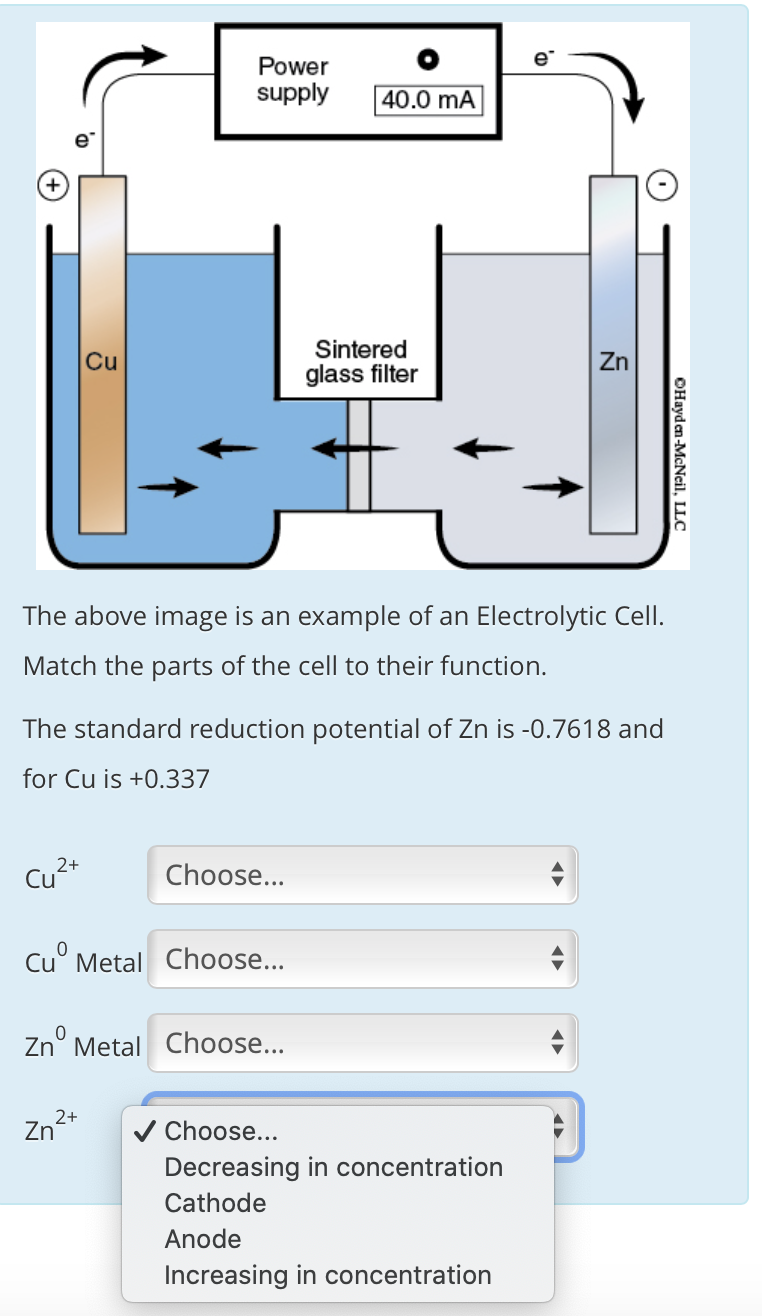
Power supply 40.0 mA Sintered Cu Zn glass filter The above image is an example of an Electrolytic Cell. Match the parts of the cell to their function. The standard reduction potential of Zn is -0.7618 and for Cu is +0.337 Cu²* Choose... Cu° Metal Choose... Zn° Metal Choose... 2+ Zn V Choose... Decreasing in concentration Cathode Anode Increasing in concentration OHayden-McNeil, LLC
Power supply 40.0 mA Sintered Cu Zn glass filter The above image is an example of an Electrolytic Cell. Match the parts of the cell to their function. The standard reduction potential of Zn is -0.7618 and for Cu is +0.337 Cu²* Choose... Cu° Metal Choose... Zn° Metal Choose... 2+ Zn V Choose... Decreasing in concentration Cathode Anode Increasing in concentration OHayden-McNeil, LLC
Chapter18: Introduction To Electrochemistry
Section: Chapter Questions
Problem 18.13QAP
Related questions
Question
Transcribed Image Text:Power
supply
40.0 mA
Sintered
Cu
Zn
glass filter
The above image is an example of an Electrolytic Cell.
Match the parts of the cell to their function.
The standard reduction potential of Zn is -0.7618 and
for Cu is +0.337
Cu²*
Choose...
Cu° Metal Choose...
Zn° Metal Choose...
2+
Zn
V Choose...
Decreasing in concentration
Cathode
Anode
Increasing in concentration
OHayden-McNeil, LLC
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning