Predict the products then write the balanced molecular and total ionic equations for the reaction that occurs when the aqueous contents of the two beakers below are added together. Sodium (Na") ions are gray. OCI Na* OH cobalt(II) chloride sodium hydroxide -------> + {NOTE: For the equations below i) Put answers in the spaces to balance the equations. ii) Do NOT use subscripts or superscripts in formula answers. Put parentheses around any polyatomic species only if there is more than one of it in the formula. Put numbers on same leve as symbols (e.g.s, the ion "NO3" should be written as (NO3)-1 or (NO3)1-; Na2co3 should be written as Na2CO3 & Ca(NO3)2 as Ca(NO3)2.) iii) Input all numerical coefficients even if it is the number "1". } (i) Molecular equation: (aq) + (aq) --> (s) + (aq) (Place #s or coefficients and formulas in the spaces) (ii) Total ionic equation: 1 Co2*(aq) + (aq) + (aq) + (aq) -> (s) + (aq) + (aq) (Place #s or coefficients and formulas in the spaces)
Predict the products then write the balanced molecular and total ionic equations for the reaction that occurs when the aqueous contents of the two beakers below are added together. Sodium (Na") ions are gray. OCI Na* OH cobalt(II) chloride sodium hydroxide -------> + {NOTE: For the equations below i) Put answers in the spaces to balance the equations. ii) Do NOT use subscripts or superscripts in formula answers. Put parentheses around any polyatomic species only if there is more than one of it in the formula. Put numbers on same leve as symbols (e.g.s, the ion "NO3" should be written as (NO3)-1 or (NO3)1-; Na2co3 should be written as Na2CO3 & Ca(NO3)2 as Ca(NO3)2.) iii) Input all numerical coefficients even if it is the number "1". } (i) Molecular equation: (aq) + (aq) --> (s) + (aq) (Place #s or coefficients and formulas in the spaces) (ii) Total ionic equation: 1 Co2*(aq) + (aq) + (aq) + (aq) -> (s) + (aq) + (aq) (Place #s or coefficients and formulas in the spaces)
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter15: Solutions
Section: Chapter Questions
Problem 42CR: 42. a. Fill in the following table as if it is a well plate and you are mixing two aqueous compounds...
Related questions
Question
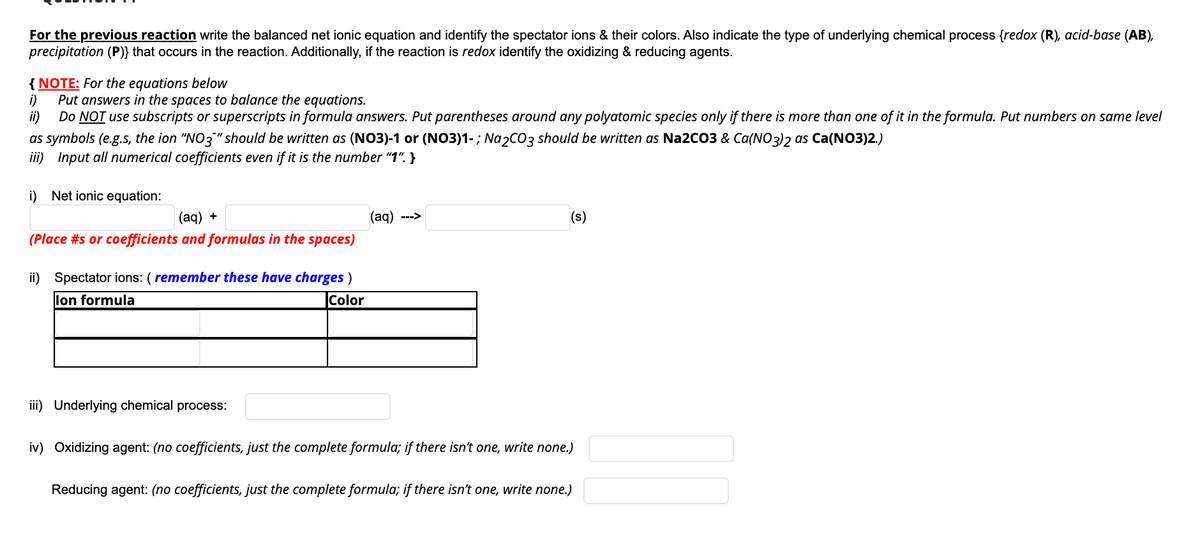
Transcribed Image Text:For the previous reaction write the balanced net ionic equation and identify the spectator ions & their colors. Also indicate the type of underlying chemical process {redox (R), acid-base (AB),
precipitation (P)} that occurs in the reaction. Additionally, if the reaction is redox identify the oxidizing & reducing agents.
{ NOTE: For the equations below
i)
Put answers in the spaces to balance the equations.
ii)
Do NOT use subscripts or superscripts in formula answers. Put parentheses around any polyatomic species only if there is more than one of it in the formula. Put numbers on same level
as symbols (e.g.s, the ion "NO3" should be written as (NO3)-1 or (NO3)1-; Na2CO3 should be written as Na2CO3 & Ca(NO3)2 as Ca(NO3)2.)
iii) Input all numerical coefficients even if it is the number "1". }
i) Net ionic equation:
(aq) +
(ag) --->
(s)
(Place #s or coefficients and formulas in the spaces)
ii) Spectator ions: ( remember these have charges )
lon formula
Color
iii) Underlying chemical process:
iv) Oxidizing agent: (no coefficients, just the complete formula; if there isn't one, write none.)
Reducing agent: (no coefficients, just the complete formula; if there isn't one, write none.)
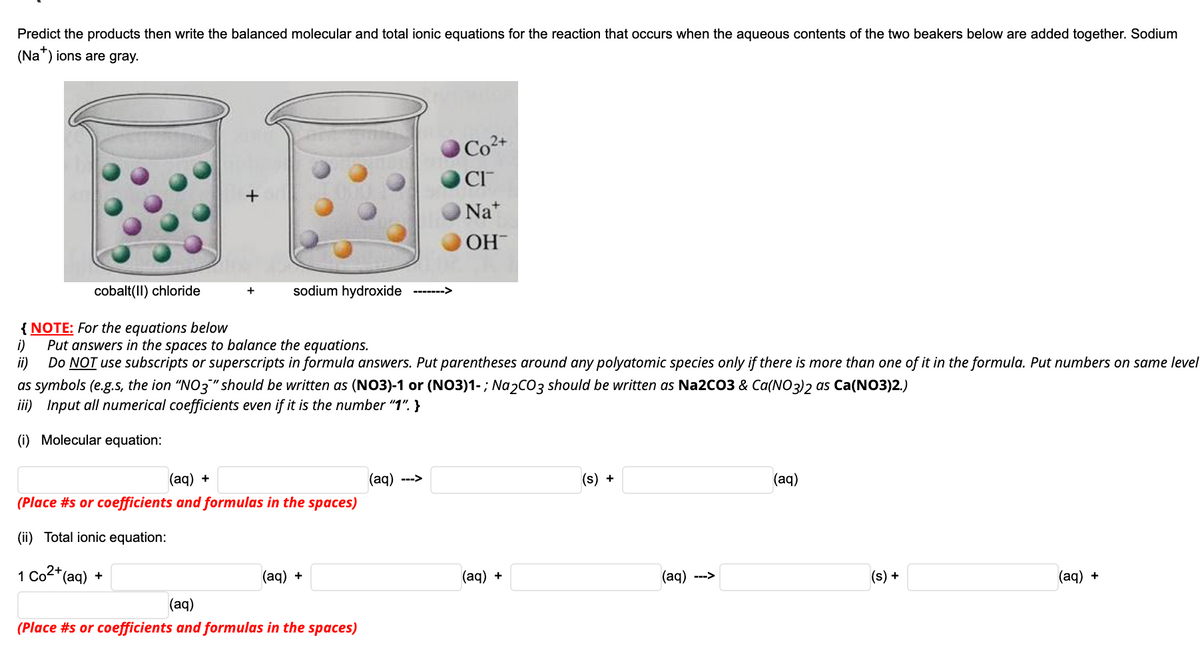
Transcribed Image Text:Predict the products then write the balanced molecular and total ionic equations for the reaction that occurs when the aqueous contents of the two beakers below are added together. Sodium
(Na") ions are gray.
Co2+
CI
ONa*
OH
cobalt(II) chloride
sodium hydroxide
+
------->
{ NOTE: For the equations below
i)
Put answers in the spaces to balance the equations.
ii)
Do NOT use subscripts or superscripts in formula answers. Put parentheses around any polyatomic species only if there is more than one of it in the formula. Put numbers on same level
as symbols (e.g.s, the ion "NO3"should be written as (NO3)-1 or (NO3)1-; Na2cO3 should be written as Na2CO3 & Ca(NO3)2 as Ca(NO3)2.)
iii) Input all numerical coefficients even if it is the number "1". }
(i) Molecular equation:
(aq) +
(aq)
(s) +
(aq)
--->
(Place #s or coefficients and formulas in the spaces)
(ii) Total ionic equation:
1 Co2*(aq) +
(aq) +
(aq) +
(ag) --->
(s) +
(aq) +
(aq)
(Place #s or coefficients and formulas in the spaces)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 5 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning