The beakers represent an atomic scale picture of the aqueous reaction between AGNO3 and NaCl. Silver (Ag*) ions are gray. What colors are used to represent NO3, Na*, and CI? Write the balanced molecular, total ionic, and net ionic equations for the reaction. a) Color of NO3 ions = b) Color of Na" ions = c) Color of CI" ions = {NOTE: For the equations below Put answers in the spaces to balance the equations. i) ii) Do NOT use subscripts or superscripts in formula answers. Put parentheses around any polyatomic species only if there is more than one of it in the formula. Put numbers on same level as symbols (e.g.s, the ion "NO3"" should be written as (NO3)-1 or (NO3)1- ; Na2CO3 should be written as Na2CO3 & Ca(NO3)2 as Ca(NO3)2.) iii) Input all numerical coefficients even if it is the number "1". } d) Molecular equation is: AGNO3(aq) + 1 NaCI(aq) --> (s) + (aq) (Place a # ONLY in left space but #s & formulas in spaces on the product side) e) Total ionic equation is (choose the correct number) I. 1 Ag*(aq) + 1 NO3 (aq) + 1 Na*(aq) + 1 CI'(aq) ---> 1 Ag*(s) + 1 CI'(s) + 1 Na*(aq) + 1 NO3 (aq) II. 1 Ag*(aq) + 1 NO3 (aq) + 1 NaCI(aq) ---> 1 A9CI(s) + 1 Na*(aq) + 1 NO3 (aq) III. 1 Ag*(aq) + 1 NO3 (aq) + 1 Na*(aq) + 1 CI"(aq) ---> 1 AgCI(s) + 1 Na*(aq) + 1 NO3 (aq) IV. 1 Ag*(aq) + 1 NO3 (aq) + 1 Na*(aq) + 1 CI(aq) ---> 1 Ag*(aq) + 1 cI'(aq) + 1 NaNO3(s) f) Net ionic equation is: (aq) + (aq) ---> (s) (Place #s or coefficients & formulas in all spaces)
The beakers represent an atomic scale picture of the aqueous reaction between AGNO3 and NaCl. Silver (Ag*) ions are gray. What colors are used to represent NO3, Na*, and CI? Write the balanced molecular, total ionic, and net ionic equations for the reaction. a) Color of NO3 ions = b) Color of Na" ions = c) Color of CI" ions = {NOTE: For the equations below Put answers in the spaces to balance the equations. i) ii) Do NOT use subscripts or superscripts in formula answers. Put parentheses around any polyatomic species only if there is more than one of it in the formula. Put numbers on same level as symbols (e.g.s, the ion "NO3"" should be written as (NO3)-1 or (NO3)1- ; Na2CO3 should be written as Na2CO3 & Ca(NO3)2 as Ca(NO3)2.) iii) Input all numerical coefficients even if it is the number "1". } d) Molecular equation is: AGNO3(aq) + 1 NaCI(aq) --> (s) + (aq) (Place a # ONLY in left space but #s & formulas in spaces on the product side) e) Total ionic equation is (choose the correct number) I. 1 Ag*(aq) + 1 NO3 (aq) + 1 Na*(aq) + 1 CI'(aq) ---> 1 Ag*(s) + 1 CI'(s) + 1 Na*(aq) + 1 NO3 (aq) II. 1 Ag*(aq) + 1 NO3 (aq) + 1 NaCI(aq) ---> 1 A9CI(s) + 1 Na*(aq) + 1 NO3 (aq) III. 1 Ag*(aq) + 1 NO3 (aq) + 1 Na*(aq) + 1 CI"(aq) ---> 1 AgCI(s) + 1 Na*(aq) + 1 NO3 (aq) IV. 1 Ag*(aq) + 1 NO3 (aq) + 1 Na*(aq) + 1 CI(aq) ---> 1 Ag*(aq) + 1 cI'(aq) + 1 NaNO3(s) f) Net ionic equation is: (aq) + (aq) ---> (s) (Place #s or coefficients & formulas in all spaces)
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter15: Acid–base Equilibria
Section: Chapter Questions
Problem 98AP
Related questions
Question
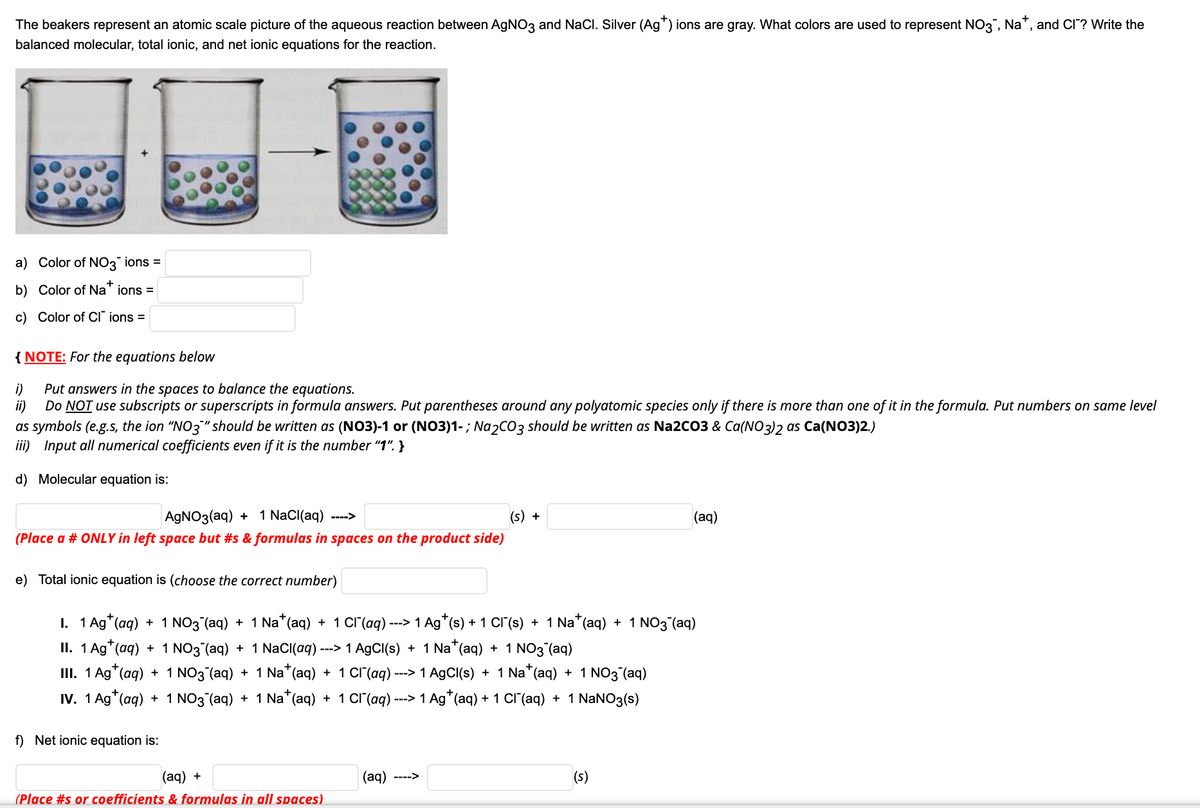
Transcribed Image Text:The beakers represent an atomic scale picture of the aqueous reaction between AGNO3 and NaCl. Silver (Ag") ions are gray. What colors are used to represent NO3, Na", and CI? Write the
balanced molecular, total ionic, and net ionic equations for the reaction.
a) Color of NO3 ions =
b) Color of Na" ions =
c) Color of CI" ions =
{ NOTE: For the equations below
Put answers in the spaces to balance the equations.
ii)
i)
Do NOT use subscripts or superscripts in formula answers. Put parentheses around any polyatomic species only if there is more than one of it in the formula. Put numbers on same level
as symbols (e.g.s, the ion "NO3" should be written as (NO3)-1 or (NO3)1-; Na2CO3 should be written as Na2CO3 & Ca(NO3)2 as Ca(NO3)2.)
iii) Input all numerical coefficients even if it is the number "1". }
d) Molecular equation is:
AGNO3(aq) + 1 NaCl(aq) ---->
(s) +
(ад)
(Place a # ONLY in left space but #s & formulas in spaces on the product side)
e) Total ionic equation is (choose the correct number)
I. 1 Ag*(aq) + 1 NO3 (aq) + 1 Na*(aq) + 1 CI"(aq) -
---> 1 Ag*(s) + 1 cr(s) + 1 Na*(aq) + 1 NO3 (aq)
II. 1 Ag*(aq) + 1 NO3 (aq) + 1 NaCl(aq) ---> 1 AgCI(s) + 1 Na*(aq) + 1 NO3 (aq)
III. 1 Ag*(aq) + 1 NO3 (aq) + 1 Na*(aq) + 1 Cr(aq) ---> 1 AgCl(s) + 1 Na*(aq) + 1 NO3 (aq)
IV. 1 Ag*(aq) + 1 NO3 (aq) + 1 Na"(aq) + 1 CI(aq) ---> 1 Ag*(aq) + 1 CI'(aq) + 1 NaN03(s)
f) Net ionic equation is:
(aq) +
(aq)
(s)
(Place #s or coefficients & formulas in all spaces)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning
