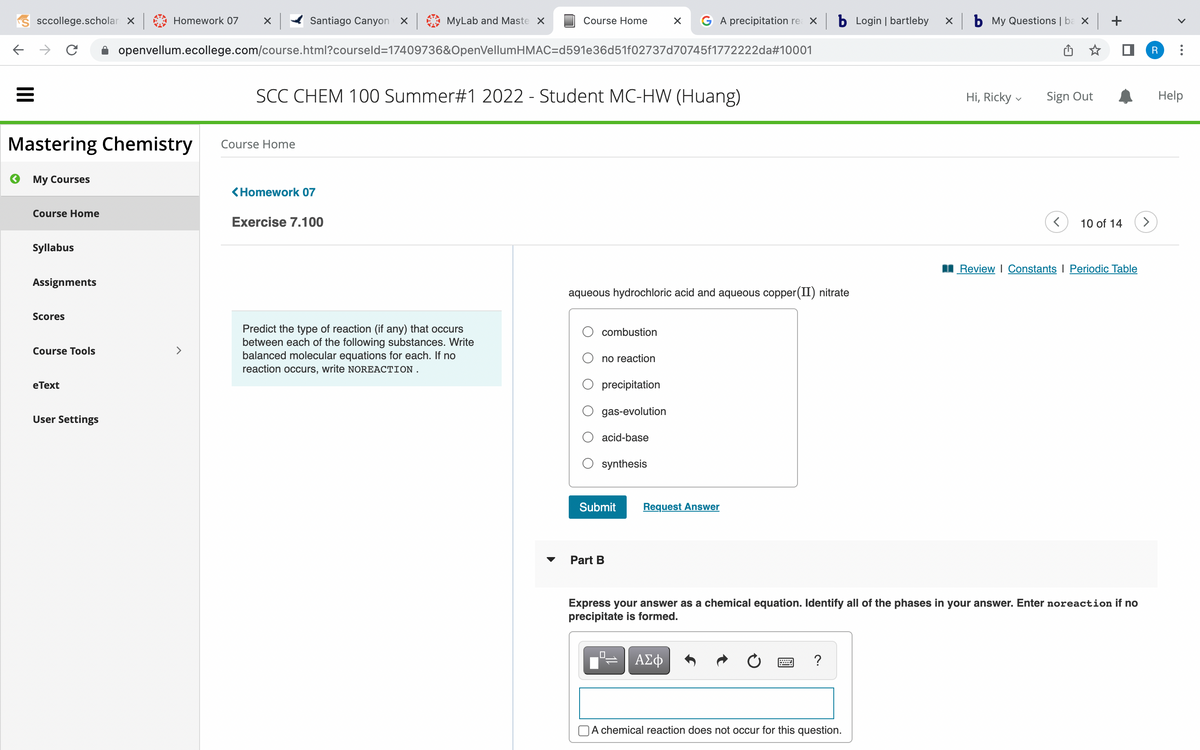
Predict the type of reaction (if any) that occurs between each of the following substances. Write balanced molecular equations for each. If no reaction occurs, write NOREACTION. aqueous hydrochloric acid and aqueous copper (II) nitrate combustion no reaction precipitation gas-evolution acid-base synthesis Submit Part B Request Answer Express your answer as a chemical equation. Identify all of the phases in your answer. Enter noreaction if no precipitate is formed. | ΑΣΦ ? Review | Constants I Periodic Table I A chemical reaction does not occur for this question.
Predict the type of reaction (if any) that occurs between each of the following substances. Write balanced molecular equations for each. If no reaction occurs, write NOREACTION. aqueous hydrochloric acid and aqueous copper (II) nitrate combustion no reaction precipitation gas-evolution acid-base synthesis Submit Part B Request Answer Express your answer as a chemical equation. Identify all of the phases in your answer. Enter noreaction if no precipitate is formed. | ΑΣΦ ? Review | Constants I Periodic Table I A chemical reaction does not occur for this question.
Chemistry for Engineering Students
3rd Edition
ISBN:9781285199023
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter1: Introduction To Chemistry
Section: Chapter Questions
Problem 1.77PAE
Related questions
Question
please solve part a and b explaining why is it that way. show your work on a piece of paper please.
Transcribed Image Text:sccollege.scholar X
← →
=
My Courses
Mastering Chemistry
Course Home
Syllabus
Assignments
Scores
Course Tools
eText
Homework 07
User Settings
X Santiago Canyon X|
openvellum.ecollege.com/course.html?courseld=17409736&OpenVellumHMAC=d591e36d51f02737d70745f1772222da#10001
Course Home
MyLab and Maste X
<Homework 07
SCC CHEM 100 Summer#1 2022 - Student MC-HW (Huang)
Exercise 7.100
Course Home
Predict the type of reaction (if any) that occurs
between each of the following substances. Write
balanced molecular equations for each. If no
reaction occurs, write NOREACTION.
aqueous hydrochloric acid and aqueous copper (II) nitrate
combustion
no reaction
precipitation
gas-evolution
acid-base
synthesis
Submit
X GA precipitation rex b Login | bartleby
Part B
0
Request Answer
ΑΣΦ
?
X
A chemical reaction does not occur for this question.
b My Questions | bax +
Hi, Ricky ✓
Express your answer as a chemical equation. Identify all of the phases in your answer. Enter noreaction if no
precipitate is formed.
Sign Out
10 of 14
Review | Constants | Periodic Table
R
Help
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning