Canvas Question 46 Å The date on a penny recovered from the floor of Ruggles MBTA stop is unreadable. The following data for this penny are then collected. Measurement Value Density of Pure Copper Density of Pure Zinc Diameter of Penny Thickness of Penny Mass of Penny 8.96 g/cm³ 7.140 g/cm³ 19.05 mm 1.52 mm 3.878 g Given the facts below, which can you conclude about this penny? Composition by Mass Minting Date of Penny Pre-1982 100% Cu 1982-Present 2.5% Cu, 97.5% Zn O the penny was minted before 1982 and contains 3.675 x 1022 atoms Cu O the penny was minted after 1982 and contains 3.483 x 1022 atoms Cu O the penny was minted before 1982 and contains 9.1187 x 1020 atoms Cu O the penny was minted after 1982 and contains 8.930 x 1020 atoms Cu MacBook Pro 4)- 8 ( 9 O
Canvas Question 46 Å The date on a penny recovered from the floor of Ruggles MBTA stop is unreadable. The following data for this penny are then collected. Measurement Value Density of Pure Copper Density of Pure Zinc Diameter of Penny Thickness of Penny Mass of Penny 8.96 g/cm³ 7.140 g/cm³ 19.05 mm 1.52 mm 3.878 g Given the facts below, which can you conclude about this penny? Composition by Mass Minting Date of Penny Pre-1982 100% Cu 1982-Present 2.5% Cu, 97.5% Zn O the penny was minted before 1982 and contains 3.675 x 1022 atoms Cu O the penny was minted after 1982 and contains 3.483 x 1022 atoms Cu O the penny was minted before 1982 and contains 9.1187 x 1020 atoms Cu O the penny was minted after 1982 and contains 8.930 x 1020 atoms Cu MacBook Pro 4)- 8 ( 9 O
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 23Q: A measurement is a quantitative observation involving both a number and a unit. What is a...
Related questions
Question
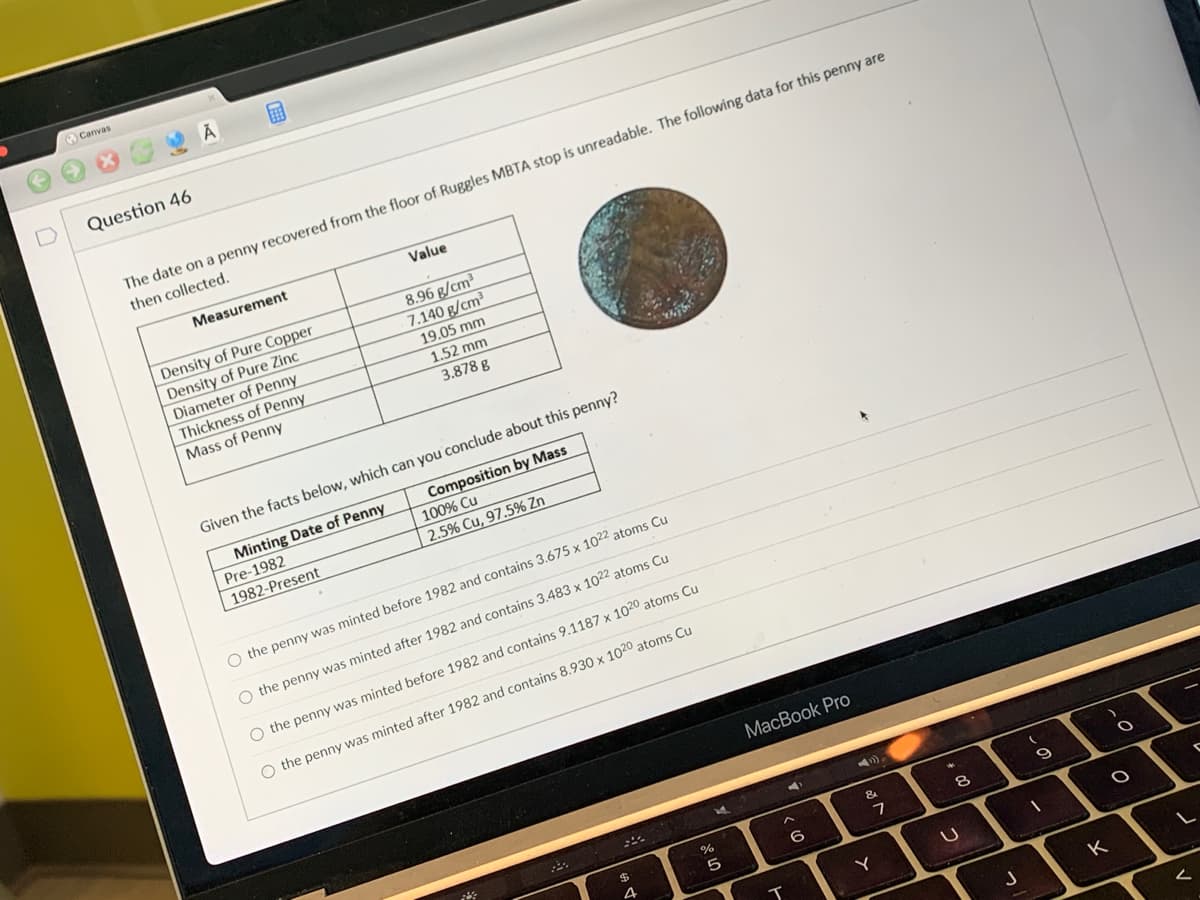
Transcribed Image Text:Canvas
Question 46
A
The date on a penny recovered from the floor of Ruggles MBTA stop is unreadable. The following data for this penny are
then collected.
Measurement
Value
Density of Pure Copper
Density of Pure Zinc
Diameter of Penny
Thickness of Penny
Mass of Penny
8.96 g/cm³
7.140 g/cm³
19.05 mm
1.52 mm
3.878 g
Given the facts below, which can you conclude about this penny?
Minting Date of Penny
Pre-1982
Composition by Mass
100% Cu
1982-Present
2.5% Cu, 97.5% Zn
O the penny was minted before 1982 and contains 3.675 x 1022 atoms Cu
O the penny was minted after 1982 and contains 3.483 x 1022 atoms Cu
O the penny was minted before 1982 and contains 9.1187 x 1020 atoms Cu
O the penny was minted after 1982 and contains 8.930 x 1020 atoms Cu
$
4
%
M
5
MacBook Pro
T
A
6
&
Y
7
1
8
U
J
9
1
O
O
K
2
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning