Preparation of standard ethylenediaminetetraacetic acid copper(II) disodium salt solution Mass of ethylenediaminetetraacetic acid copper(II) disodium salt (± 0.002 g.) 0.613 g Molar mass of ethylenediaminetetraacetic acid copper(II) disodium salt = 397.74 g mol–1. Show your calculations here (with appropriate number of significant digits and units in final result) amount (mol) of solute volume of solution (litres) Molarity Concentration of ethylenediaminetetraacetic acid copper(II) disodium salt =
Preparation of standard ethylenediaminetetraacetic acid copper(II) disodium salt solution Mass of ethylenediaminetetraacetic acid copper(II) disodium salt (± 0.002 g.) 0.613 g Molar mass of ethylenediaminetetraacetic acid copper(II) disodium salt = 397.74 g mol–1. Show your calculations here (with appropriate number of significant digits and units in final result) amount (mol) of solute volume of solution (litres) Molarity Concentration of ethylenediaminetetraacetic acid copper(II) disodium salt =
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 1RQ: Define and explain the differences between the following terms. a. law and theory b. theory and...
Related questions
Question
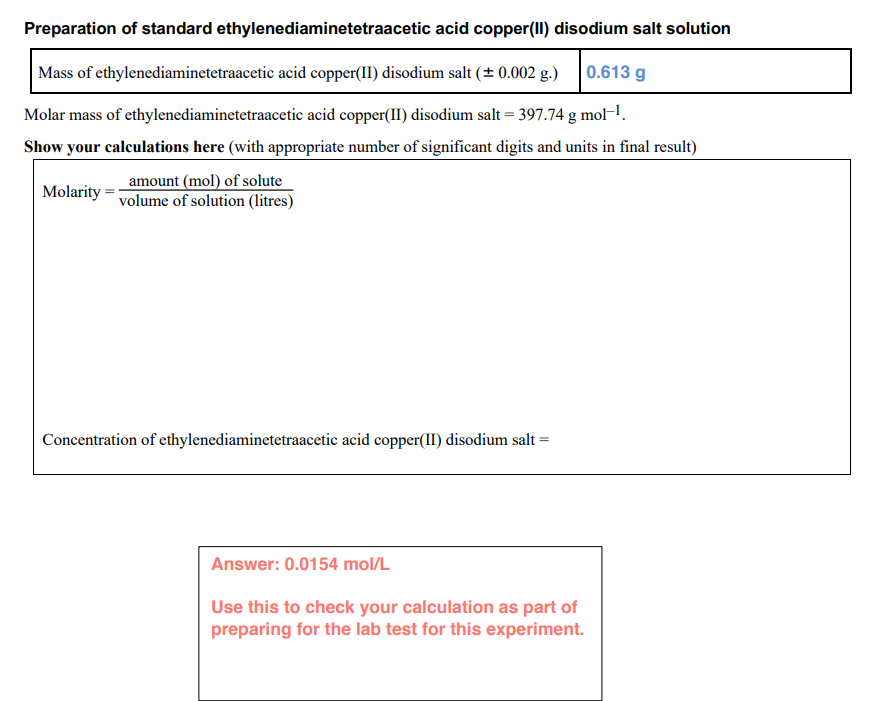
Transcribed Image Text:Preparation of standard ethylenediaminetetraacetic acid copper(II) disodium salt solution
Mass of ethylenediaminetetraacetic acid copper(II) disodium salt (+ 0.002 g.)
|0.613 g
Molar mass of ethylenediaminetetraacetic acid copper(II) disodium salt = 397.74 g mol-.
Show your calculations here (with appropriate number of significant digits and units in final result)
amount (mol) of solute
volume of solution (litres)
Molarity
Concentration of ethylenediaminetetraacetic acid copper(II) disodium salt =
Answer: 0.0154 mol/L
Use this to check your calculation as part of
preparing for the lab test for this experiment.

Transcribed Image Text:Method
Note: In steps 1 and 2 you will use the technique of weighing by difference to measure the exact mass of the sample of the
ethylenediaminetetraacetic acid copper(II) disodium salt. This technique is described in the 'Techniques' section above and
is available to watch as a chemistry skills video online (link available in Moodle).
Weigh the ethylenediaminetetraacetic acid copper(II) disodium salt
1. If the sample tube on the balance is not already about one-third filled, fill it to about 1/3 of its height with
ethylenediaminetetraacetic acid copper(II) disodium salt. Place a plastic spatula in the sample tube with the
ethylenediaminetetraacetic acid copper(II) disodium salt and place on an analytical balance. Tare the balance so that it
reads zero.
Warning: After carrying out step 1, do not place the spatula on the bench, as this may result in a loss of sample, and
hence an incorrect determination of the mass of the sample.
2. Using the spatula, transfer without loss (i.e. don't spill any), some of the solid from the sample tube into a 100 or 150
mL beaker. Replace the spatula and reweigh the sample tube and spatula. Repeat until you have about 0.6 g in your
beaker. Record on the results page the exact mass of the ethylenediaminetetraacetic acid copper(II) disodium salt
weighed. The balance reading will be negative since the tube contains less solid for the second reading than the first.
Dissolve the ethylenediaminetetraacetic acid copper(II) disodium salt in water in a beaker
3. Add about 50 mL of water to the beaker to dissolve the ethylenediaminetetraacetic acid copper(II) disodium salt.
Carefully swirl the mixture to aid dissolution. Do not spill the solution!
Warning: Make sure you use the correct technique for using a volumetric flask in the steps below.
Transfer the solution into a volumetric flask and rinse the beaker into the volumetric flask
4. Using a plastic funnel, transfer the solution into a clean 100 mL volumetric flask being careful not to lose any. Rinse
the beaker and funnel with a jet of water from a wash bottle several times to ensure all the ethylenediaminetetraacetic
acid copper(II) disodium salt is transferred to the volumetric flask.
Carefully add water to volumetric flask to the mark and mix solution in the flask
5. Add water carefully from a beaker until the water level is 1 to 2 cm below the graduation mark on the neck of the
volumetric flask.
6. Using a squash pipette, add water drop by drop until the bottom of the meniscus is in line with the graduation mark on
the flask (warning: if the bottom of the meniscus goes over the graduation mark, you have to start again). Stopper the
flask and mix thoroughly (this is important!). While holding the stopper in place, tum the flask upside down several
times.
7. Calculate the concentration of your ethylenediaminetetraacetic acid copper(II) disodium salt solution, based on the actual
mass weighed out.
Preparing a sample of your standard solution for analysis
8. Rinse out a cuvette using some of the standard solution that you have just made (a new clean squash pipette should be
used for the transfer). Repeat, rinsing out the cuvette a second time. Next fill the cuvette to % full with the standard
solution and take this to your demonstrator.
9. Your demonstrator will measure the light absorbance of your solution, which you should record and then complete the
required calculations. The light absorbance depends on concentration so this is a way to determine how accurate you
have been in weighing the solute and using the volumetric flask.
Clean up
10. Dispose of all copper solutions into the heavy metals chemical waste container in the fume cupboard. Wash and clean
all glassware and apparatus that you have used with detergent provided at the sinks and return them to where you got
them and ask vour demonstrator to check vour locker.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9780078021558
Author:
Janice Gorzynski Smith Dr.
Publisher:
McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Elementary Principles of Chemical Processes, Bind…
Chemistry
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY