Problem 13.34 outlines a general method for the preparation of cis- or trans-disubstituted epoxides. Using that method, identify what reagents you would use to prepare the following epoxide from acetylene: H "Et The transformation above can be performed with some reagent or combination of the reagents listed below. Give the necessary reagent(s) in the correct order, as a string of letters (without spaces or punctuation, such as "EBF"). If there is more than one correct solution, provide just one answer. B D. DMP or PCC H2, Pt EtBr PHCH2BR F H H2SO4, H20, HgsO4 МСРВА (RCO3H) Na, NH3 (/) H2, Lindlar's cat. K TSCI, py PhBr NANH2 1) EtMgBr; 2) H,0+
Ionic Equilibrium
Chemical equilibrium and ionic equilibrium are two major concepts in chemistry. Ionic equilibrium deals with the equilibrium involved in an ionization process while chemical equilibrium deals with the equilibrium during a chemical change. Ionic equilibrium is established between the ions and unionized species in a system. Understanding the concept of ionic equilibrium is very important to answer the questions related to certain chemical reactions in chemistry.
Arrhenius Acid
Arrhenius acid act as a good electrolyte as it dissociates to its respective ions in the aqueous solutions. Keeping it similar to the general acid properties, Arrhenius acid also neutralizes bases and turns litmus paper into red.
Bronsted Lowry Base In Inorganic Chemistry
Bronsted-Lowry base in inorganic chemistry is any chemical substance that can accept a proton from the other chemical substance it is reacting with.
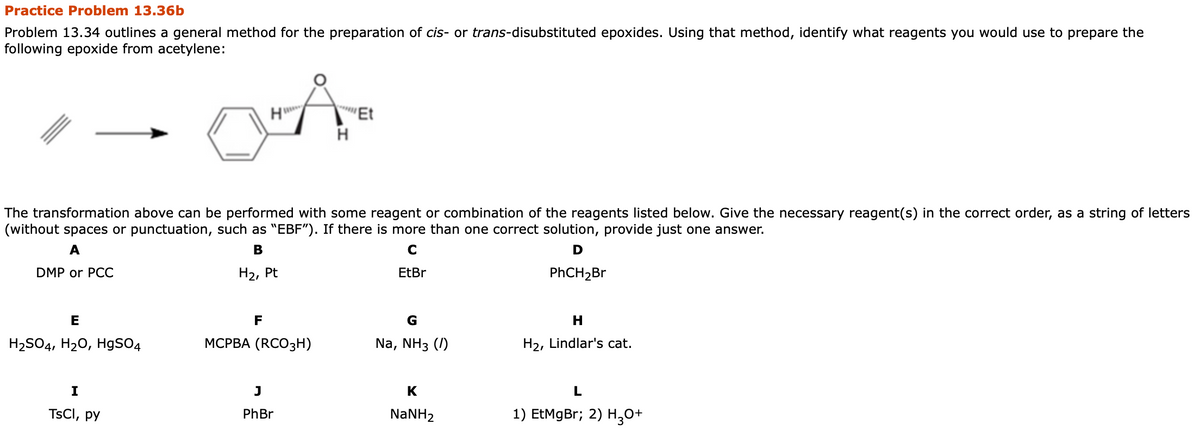
General outline for preparation of trans-disubstituted epoxide
| Step | Reagent used | |
| Step1 |
NaNH2 and RCH2Br R can be phenyl and some other alkyl or aryl Substituent |
Acidic Proton abstraction by base and Substitution reaction Mono substituted terminal Alkyne will form
|
| Step 2 |
NaNH2 and R'Br R' can be ethyl methyl |
Another Acidic Proton of acetylene abstraction by base and Substitution reaction with R'Br Di substituted Alkyne will form |
| Step 3 | Na / NH3(l) | Gives Trans alkene |
| Step4 | MCPBA (RCO3H) |
trans-disubstituted Epoxide formation
|
General outline for preparation of cis -disubstituted epoxide
| Step | Reagent used | |
| Step1 |
NaNH2 and RCH2Br R can be phenyl and some other alkyl or aryl Substituent |
Acidic Proton abstraction by base and Substitution reaction Mono substituted terminal Alkyne will form
|
| Step 2 |
NaNH2 and R'Br R' can be ethyl methyl etc. |
Another Acidic Proton of acetylene abstraction by base and Substitution reaction with R'Br Di substituted Alkyne will form |
| Step 3 | H2 Lindlar Catalyst | Gives cis alkene |
| Step4 | MCPBA (RCO3H) | Cis-disubstituted Epoxide formation |
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images


