Problem 20.20 One mole of monatomic gas undergoes a Carnot cycle with TH = 380 °C and T, = 220 °C. The initial pressure is Pa = 8.5 atm (see Figure 20-7 in the textbook). During the isothermal expansion, the volume doubles. Part A Find the values of the pressure at the points a, b, c, and d. Express your answers using two significant figures. Enter your answers numerically separated by commas. ANSWER: Pa, P, Pe, Pa = 6.3-10-3, 12.608, 19.22, 9.61 atm Incorrect; Try Again; 5 attempts remaining Problem 20.20 One mole of monatomic gas undergoes a Carnot cycle with TH = 380 °C and TL = 220 °C. The initial pressure is Pa = 8.5 atm (see Figure 20-7 in the textbook). During the isothermal expansion, the volume doubles.
Problem 20.20 One mole of monatomic gas undergoes a Carnot cycle with TH = 380 °C and T, = 220 °C. The initial pressure is Pa = 8.5 atm (see Figure 20-7 in the textbook). During the isothermal expansion, the volume doubles. Part A Find the values of the pressure at the points a, b, c, and d. Express your answers using two significant figures. Enter your answers numerically separated by commas. ANSWER: Pa, P, Pe, Pa = 6.3-10-3, 12.608, 19.22, 9.61 atm Incorrect; Try Again; 5 attempts remaining Problem 20.20 One mole of monatomic gas undergoes a Carnot cycle with TH = 380 °C and TL = 220 °C. The initial pressure is Pa = 8.5 atm (see Figure 20-7 in the textbook). During the isothermal expansion, the volume doubles.
Chapter4: The Second Law Of Thermodynamics
Section: Chapter Questions
Problem 86CP: A cylinder contains 500 g of helium at 120 atm and 20 . The valve is leaky, and all the gas slowly...
Related questions
Question
Please help me, double check your answers, previous tutors got it wrong, and make sure you provide all 4 values. THIS IS THE FULL QUESTION,
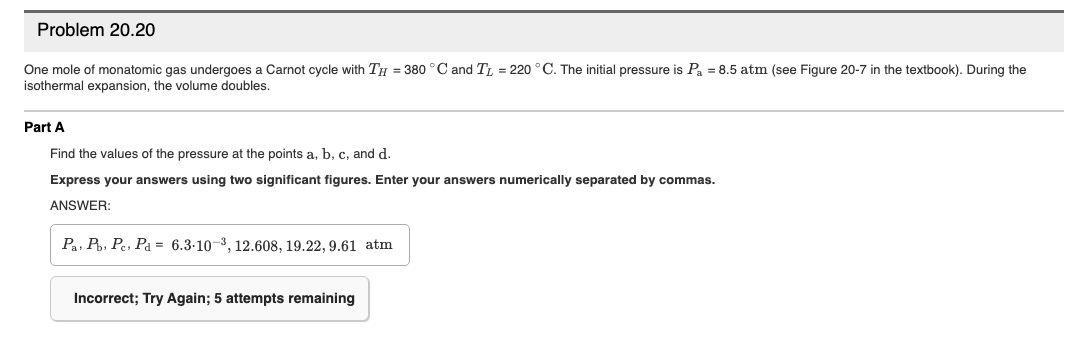
Transcribed Image Text:Problem 20.20
One mole of monatomic gas undergoes a Carnot cycle with TH = 380 °C and T, = 220 °C. The initial pressure is Pa = 8.5 atm (see Figure 20-7 in the textbook). During the
isothermal expansion, the volume doubles.
Part A
Find the values of the pressure at the points a, b, c, and d.
Express your answers using two significant figures. Enter your answers numerically separated by commas.
ANSWER:
Pa, P, Pe, Pa = 6.3-10-3, 12.608, 19.22, 9.61 atm
Incorrect; Try Again; 5 attempts remaining

Transcribed Image Text:Problem 20.20
One mole of monatomic gas undergoes a Carnot cycle with TH = 380 °C and TL = 220 °C. The initial pressure is Pa = 8.5 atm (see Figure 20-7 in the textbook). During the
isothermal expansion, the volume doubles.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 5 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Recommended textbooks for you


Physics for Scientists and Engineers: Foundations…
Physics
ISBN:
9781133939146
Author:
Katz, Debora M.
Publisher:
Cengage Learning

College Physics
Physics
ISBN:
9781938168000
Author:
Paul Peter Urone, Roger Hinrichs
Publisher:
OpenStax College


Physics for Scientists and Engineers: Foundations…
Physics
ISBN:
9781133939146
Author:
Katz, Debora M.
Publisher:
Cengage Learning

College Physics
Physics
ISBN:
9781938168000
Author:
Paul Peter Urone, Roger Hinrichs
Publisher:
OpenStax College