Problem Solving Using the blank periodic table, locate the following elements (represented by capital letters) with corresponding description. A- has the lowest electronegativity value B- has the largest atomic size in group 3A C-has the highest first ionization energy D- has the lowest electron affinity in period 4 E- has five valence electrons and it is in period 2 F-is the lightest of the alkaline Earth metals emsM gelA8 lopni hsM M erlos Ova of ebnogdemdo enl J9erle vivitoe eda vlivis ed swa A 7ebnim lepoLi neM emet thuoco erb divit oevide
Problem Solving Using the blank periodic table, locate the following elements (represented by capital letters) with corresponding description. A- has the lowest electronegativity value B- has the largest atomic size in group 3A C-has the highest first ionization energy D- has the lowest electron affinity in period 4 E- has five valence electrons and it is in period 2 F-is the lightest of the alkaline Earth metals emsM gelA8 lopni hsM M erlos Ova of ebnogdemdo enl J9erle vivitoe eda vlivis ed swa A 7ebnim lepoLi neM emet thuoco erb divit oevide
Introductory Chemistry: An Active Learning Approach
6th Edition
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Mark S. Cracolice, Ed Peters
Chapter11: Atomic Theory :the Quantum Model Of The Atom
Section: Chapter Questions
Problem 85E
Related questions
Question
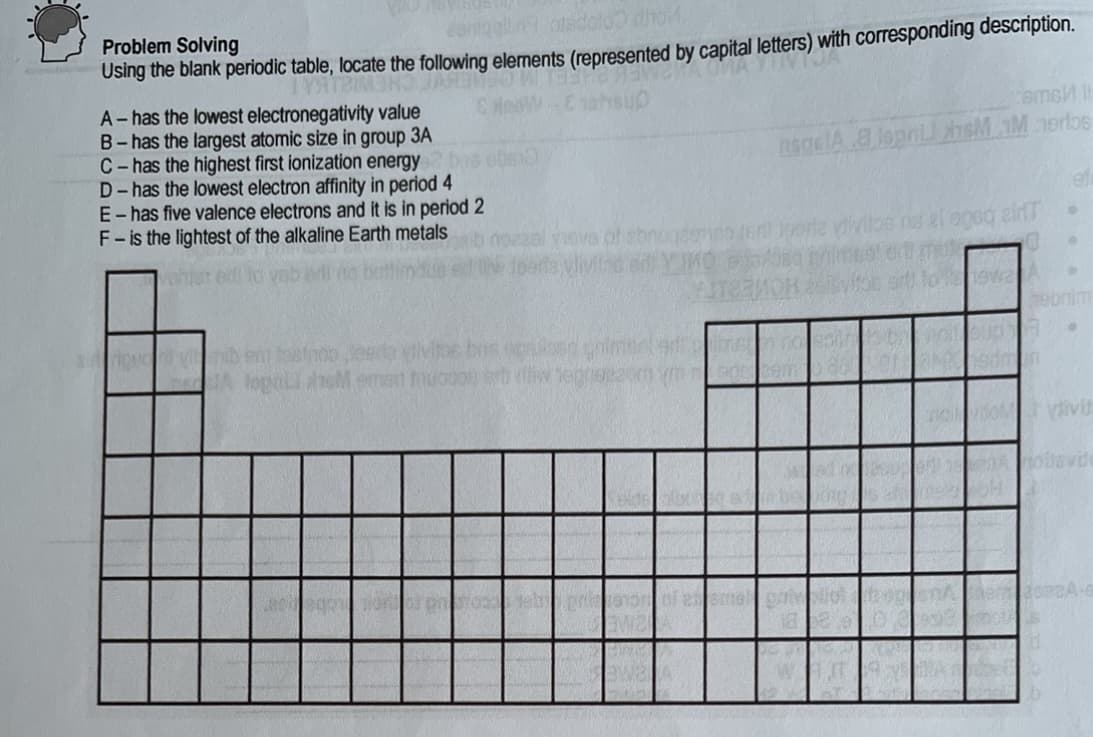
Transcribed Image Text:Problem Solving
Using the blank periodic table, locate the following elements (represented by capital letters) with corresponding description.
A- has the lowest electronegativity value
B- has the largest atomic size in group 3A
C-has the highest first ionization energy
D-has the lowest electron affinity in period 4
E- has five valence electrons and it is in period 2
F- is the lightest of the alkaline Earth metals
ebno
en 9eda ywilos ns zi ogeg airT
Iivitos bris opisd
A lepoLi neM emen huooo er thw
Kivit
noievite
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co