Problem The molecular scenes below represent equal volumes of four HA/A¯ buffers. (HA is blue and green, A¯ is green, and other ions and water are not shown.) 1 2 4 (a) Which buffer has the highest pH? (b) Which buffer has the greatest capacity? (c) Should we add a small amount of concentrated strong acid or strong base to convert sample 1 to sample 2 (assuming no volume change)?
Problem The molecular scenes below represent equal volumes of four HA/A¯ buffers. (HA is blue and green, A¯ is green, and other ions and water are not shown.) 1 2 4 (a) Which buffer has the highest pH? (b) Which buffer has the greatest capacity? (c) Should we add a small amount of concentrated strong acid or strong base to convert sample 1 to sample 2 (assuming no volume change)?
Chapter8: Polyfunctional Acids And Bases
Section: Chapter Questions
Problem 6P
Related questions
Question
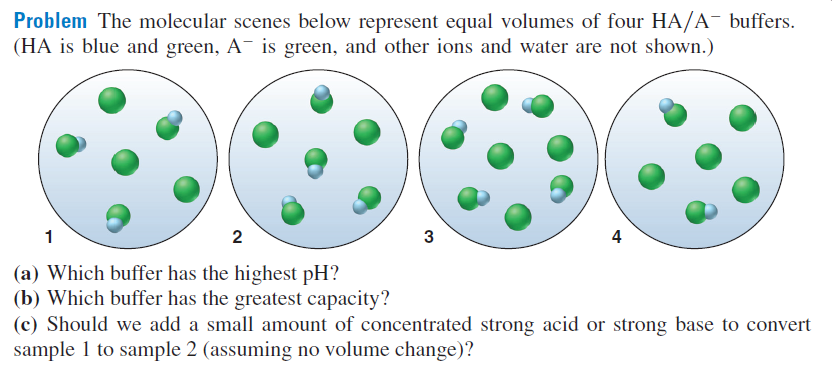
Transcribed Image Text:Problem The molecular scenes below represent equal volumes of four HA/A¯ buffers.
(HA is blue and green, A¯ is green, and other ions and water are not shown.)
1
2
4
(a) Which buffer has the highest pH?
(b) Which buffer has the greatest capacity?
(c) Should we add a small amount of concentrated strong acid or strong base to convert
sample 1 to sample 2 (assuming no volume change)?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you



Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning



Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning