Procaine (its hydrochloride is marketed as Novocain) was one of the first local anesthet- ics for infiltration and regional anesthesia. See "Chemical Connections: From Cocaine to Procaine and Beyond."According to the following retrosynthetic scheme, procaine can be synthesized from 4-aminobenzoic acid, ethylene oxide, and diethylamine as sources of carbon atoms. HO. + HO NEt, H,N H,N' Procaine 4-Aminobenzoic acid Et,NH Ethylene oxide Diethylamine Provide reagents and experimental conditions to carry out the synthesis of procaine from these three compounds.
Procaine (its hydrochloride is marketed as Novocain) was one of the first local anesthet- ics for infiltration and regional anesthesia. See "Chemical Connections: From Cocaine to Procaine and Beyond."According to the following retrosynthetic scheme, procaine can be synthesized from 4-aminobenzoic acid, ethylene oxide, and diethylamine as sources of carbon atoms. HO. + HO NEt, H,N H,N' Procaine 4-Aminobenzoic acid Et,NH Ethylene oxide Diethylamine Provide reagents and experimental conditions to carry out the synthesis of procaine from these three compounds.
Organic Chemistry
8th Edition
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Chapter18: Functional Derivatives Of Carboxylic Acids
Section: Chapter Questions
Problem 18.42P
Related questions
Question
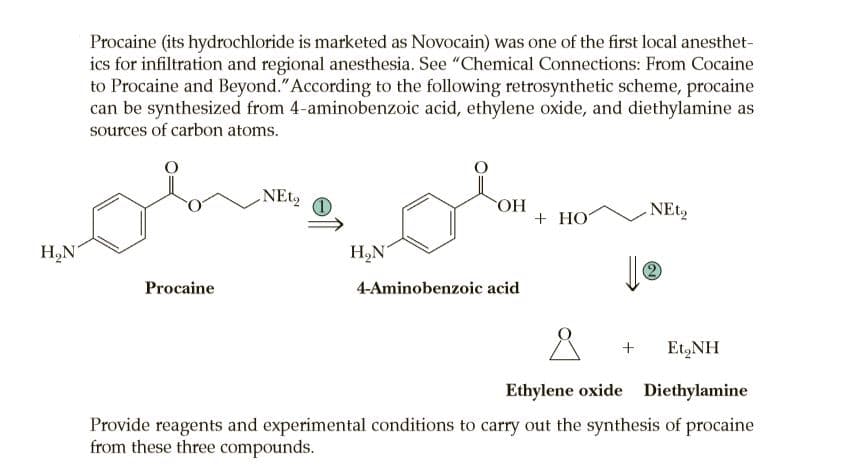
Transcribed Image Text:Procaine (its hydrochloride is marketed as Novocain) was one of the first local anesthet-
ics for infiltration and regional anesthesia. See "Chemical Connections: From Cocaine
to Procaine and Beyond."According to the following retrosynthetic scheme, procaine
can be synthesized from 4-aminobenzoic acid, ethylene oxide, and diethylamine as
sources of carbon atoms.
HO.
+ HO
NEt,
H,N
H,N'
Procaine
4-Aminobenzoic acid
Et,NH
Ethylene oxide Diethylamine
Provide reagents and experimental conditions to carry out the synthesis of procaine
from these three compounds.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning