Prof Adam and his colleagues synthesized compound E, with th formula CSHCI. They wanted to identify the chemical properties of this compound by conducting a series of chemical tests. Compound E produced compound F when it reacted with sodium hydroxide in the presence of water and reflux. In the presence of ultraviolet light, compound G reacts with halogen gas to form compound E. Compound E is formed when compound H undergoes addition reactions involving hydrogen chloride. When compound E reacts with aqueous ammonia in the presence of heat, compound I is formed. Draw the possible skeletal structures of compound F to I. Identify the IUPAC nomenclature of compounds F, G, H, and I.
Prof Adam and his colleagues synthesized compound E, with th formula CSHCI. They wanted to identify the chemical properties of this compound by conducting a series of chemical tests. Compound E produced compound F when it reacted with sodium hydroxide in the presence of water and reflux. In the presence of ultraviolet light, compound G reacts with halogen gas to form compound E. Compound E is formed when compound H undergoes addition reactions involving hydrogen chloride. When compound E reacts with aqueous ammonia in the presence of heat, compound I is formed. Draw the possible skeletal structures of compound F to I. Identify the IUPAC nomenclature of compounds F, G, H, and I.
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter7: Bonding In Organic Molecules
Section: Chapter Questions
Problem 40AP: Consider the following proposed structures for benzene, each of which is consistent with the...
Related questions
Question
100%
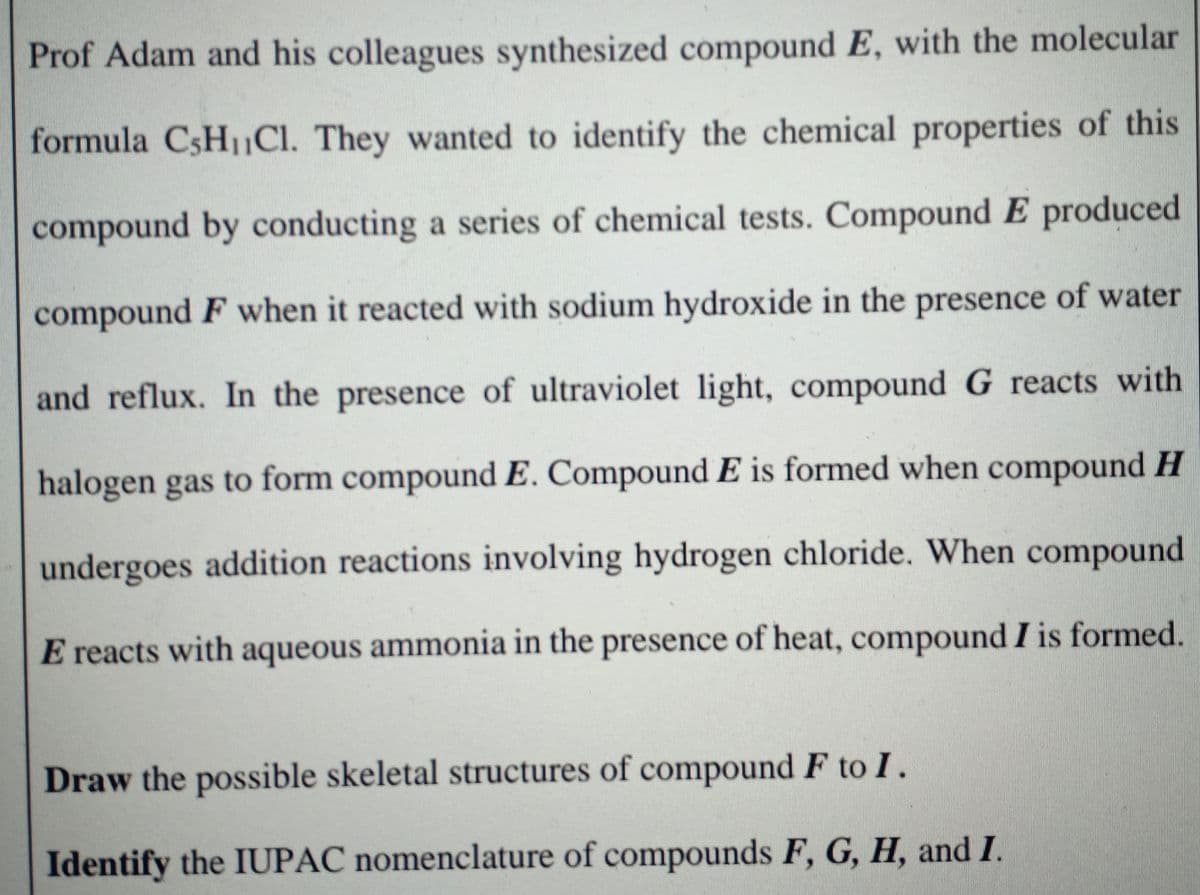
Transcribed Image Text:Prof Adam and his colleagues synthesized compound E, with the molecular
formula CSH1CI. They wanted to identify the chemical properties of this
compound by conducting a series of chemical tests. Compound E produced
compound F when it reacted with sodium hydroxide in the presence of water
and reflux. In the presence of ultraviolet light, compound G reacts with
halogen gas to form compound E. Compound E is formed when compound H
undergoes addition reactions involving hydrogen chloride. When compound
E reacts with aqueous ammonia in the presence of heat, compound I is formed.
Draw the possible skeletal structures of compound F to I.
Identify the IUPAC nomenclature of compounds F, G, H, and I.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning