Prove that carnot engine efficiency can never be one
Carnot heat engine is a theoretical engine which operates between two temperatures on a Carnot cycle.
It helps in converting the heat into work with maximum possible efficiency possible
The cycle involves 4 reversible process which are
1) Isothermal expansion
2) Adiabatic expansion
3) Isothermal compression
4) Adiabatic compression
The P v/s V cycle for a Carnot heat engine looks like as shown below
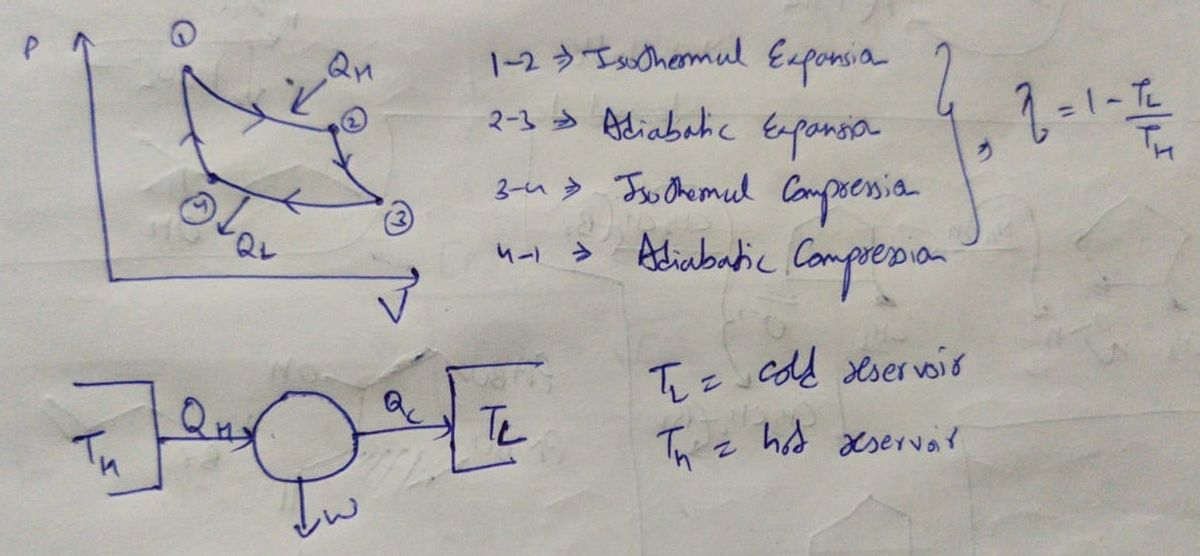
The necessary thermodynamic quantities involved in each step are
1) process (1-2) i.e Isothermal Expansion : Since the process is isothermal => Temperature is constant
hence internal energy change is 0
=> QH = -W = nRTH X ln(V2 / V1 )
where QH = heat of the process, W = work
n = moles of gas, R = gas constant and TH = hot reservoir temperature
V2 = final volume after expansion and V1 = initial volume before expansion
2) process (2-3) i.e Adiabatic Expansion : Since the process is Adiabatic => Q = 0
=> W = ΔU = nCvΔT
where ΔU = change in internal energy , W = work
n = moles of gas, Cv = specific heat for the gas and ΔT = change in temperature = TL - TH
TH = hot reservoir temperature and TL = cold reservoir temperature
Step by step
Solved in 7 steps with 1 images




